Region:Middle East
Author(s):Geetanshi
Product Code:KRAD7179
Pages:83
Published On:December 2025
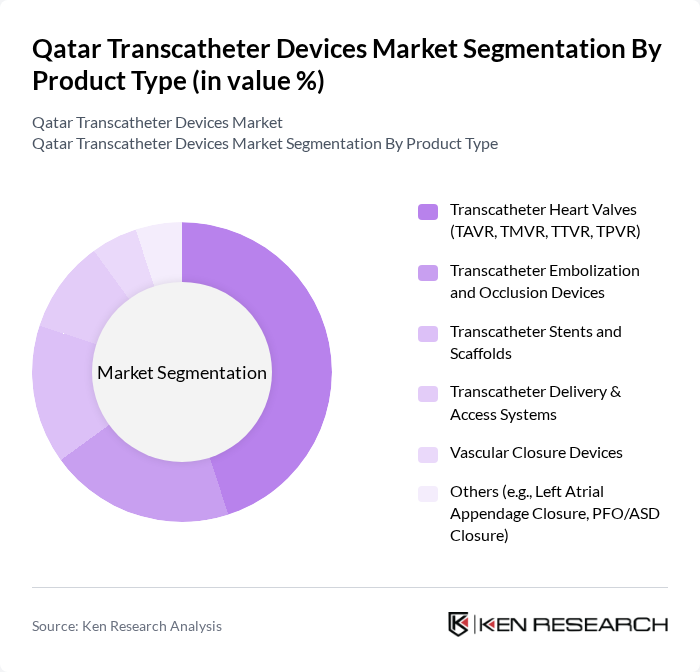
By Product Type:The product type segmentation includes various categories such as Transcatheter Heart Valves (TAVR, TMVR, TTVR, TPVR), Transcatheter Embolization and Occlusion Devices, Transcatheter Stents and Scaffolds, Transcatheter Delivery & Access Systems, Vascular Closure Devices, and Others (e.g., Left Atrial Appendage Closure, PFO/ASD Closure). Among these, Transcatheter Heart Valves are leading the market due to their critical role in treating severe aortic stenosis and other structural heart diseases. The increasing adoption of these devices in hospitals and specialty centers is driven by their proven efficacy and safety profiles.
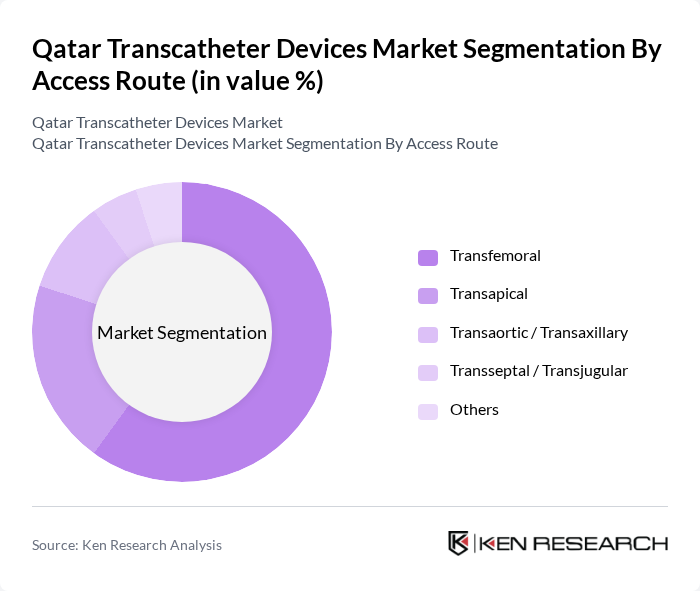
By Access Route:The access route segmentation includes Transfemoral, Transapical, Transaortic / Transaxillary, Transseptal / Transjugular, and Others. The Transfemoral approach is the most commonly used due to its minimally invasive nature and lower complication rates. This method allows for easier access to the heart, making it a preferred choice among healthcare professionals for various transcatheter procedures.
The Qatar Transcatheter Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Abbott Laboratories, Boston Scientific Corporation, Edwards Lifesciences Corporation, Terumo Corporation, Johnson & Johnson MedTech (including Cordis legacy portfolio), Biotronik SE & Co. KG, W. L. Gore & Associates, Inc., Cook Medical LLC, Merit Medical Systems, Inc., Philips Image Guided Therapy (Philips Healthcare), Siemens Healthineers AG, Lifetech Scientific Corporation, Lepu Medical Technology (Beijing) Co., Ltd., Venus Medtech (Hangzhou) Inc. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Qatar transcatheter devices market appears promising, driven by technological advancements and an increasing focus on patient-centered care. As healthcare facilities expand and invest in modern technologies, the integration of digital health solutions is expected to enhance patient outcomes. Furthermore, the growing emphasis on personalized medicine will likely lead to tailored treatment options, fostering innovation and collaboration among local and international medical device companies, ultimately improving healthcare delivery in Qatar.
| Segment | Sub-Segments |
|---|---|
| By Product Type | Transcatheter Heart Valves (TAVR, TMVR, TTVR, TPVR) Transcatheter Embolization and Occlusion Devices Transcatheter Stents and Scaffolds Transcatheter Delivery & Access Systems Vascular Closure Devices Others (e.g., Left Atrial Appendage Closure, PFO/ASD Closure) |
| By Access Route | Transfemoral Transapical Transaortic / Transaxillary Transseptal / Transjugular Others |
| By Clinical Application | Structural Heart Disease (Aortic, Mitral, Tricuspid, Pulmonary) Coronary Artery Disease Congenital Heart Defects Peripheral Vascular Disease Others |
| By End-User | Tertiary Care Hospitals Cardiac Specialty Centers Ambulatory Surgical Centers / Day-care Cath Labs Research & Academic Institutions Others |
| By Distribution Channel | Direct Sales to Hospitals & Health Systems Local Distributors / Agents Group Purchasing & Tender-Based Procurement Others |
| By Patient Demographics | Age Group (Pediatric, Adult, Geriatric) Gender (Male, Female) Nationality (Qatari, Expatriate) Others |
| By Technology / Mechanism | Balloon-Expandable Technology Self-Expanding Technology Bioresorbable / Next-Generation Platforms Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Cardiovascular Hospitals | 100 | Cardiologists, Interventional Radiologists |
| Medical Device Distributors | 75 | Sales Managers, Product Specialists |
| Healthcare Procurement Departments | 60 | Procurement Officers, Supply Chain Managers |
| Regulatory Bodies | 40 | Regulatory Affairs Specialists, Policy Makers |
| Patient Advocacy Groups | 40 | Patient Representatives, Health Advocates |
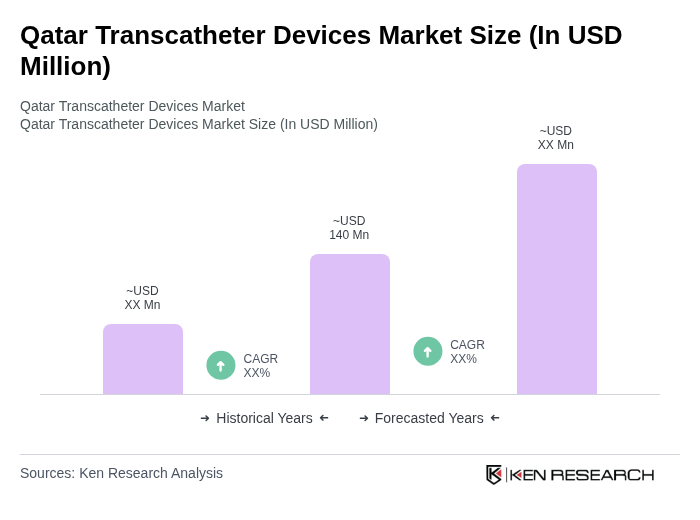
The Qatar Transcatheter Devices Market is valued at approximately USD 140 million, reflecting a significant growth driven by the rising prevalence of cardiovascular diseases and advancements in minimally invasive surgical techniques.