Region:Middle East
Author(s):Geetanshi
Product Code:KRAC9427
Pages:81
Published On:November 2025
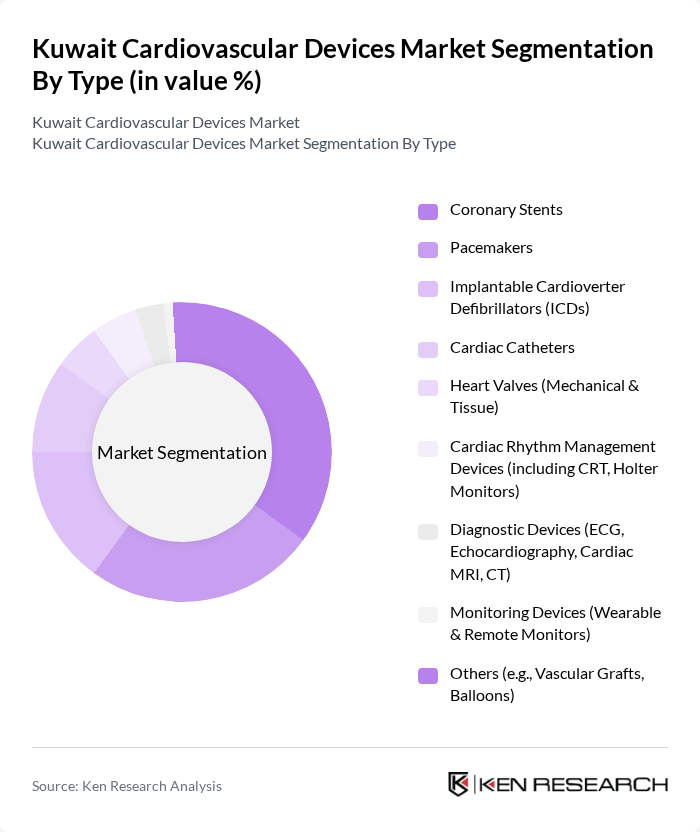
By Type:The market is segmented into various types of cardiovascular devices, including coronary stents, pacemakers, implantable cardioverter defibrillators (ICDs), cardiac catheters, heart valves (mechanical & tissue), cardiac rhythm management devices (including CRT and Holter monitors), diagnostic devices (ECG, echocardiography, cardiac MRI, CT), monitoring devices (wearable & remote monitors), and others (e.g., vascular grafts, balloons). Among these, coronary stents and pacemakers are the leading subsegments due to their widespread use in treating coronary artery diseases and arrhythmias, respectively. The increasing incidence of heart diseases and technological advancements in these devices contribute to their dominance.
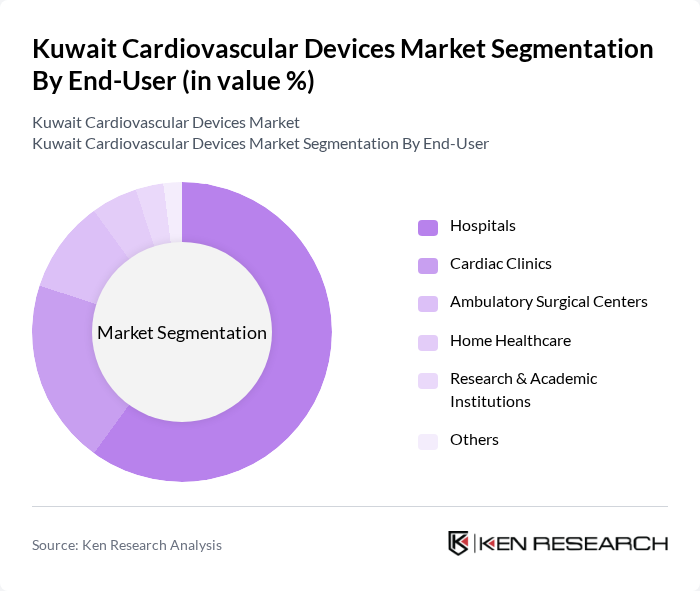
By End-User:The end-user segmentation includes hospitals, cardiac clinics, ambulatory surgical centers, home healthcare, research & academic institutions, and others. Hospitals are the primary end-users, accounting for a significant share of the market due to their comprehensive facilities and specialized departments for cardiovascular care. The increasing number of cardiac procedures performed in hospitals, coupled with the growing demand for advanced medical technologies, drives the market in this segment.
The Kuwait Cardiovascular Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Boston Scientific Corporation, Abbott Laboratories, Johnson & Johnson (Biosense Webster & Cordis), Siemens Healthineers AG, Philips Healthcare (Koninklijke Philips N.V.), Edwards Lifesciences Corporation, Biotronik SE & Co. KG, Terumo Corporation, B. Braun Melsungen AG, Stryker Corporation, Cook Medical LLC, St. Jude Medical (now part of Abbott), Cardiovascular Systems, Inc., AtriCure, Inc., GE HealthCare, W. L. Gore & Associates, Inc., MicroPort Scientific Corporation, Lepu Medical Technology, Shree Pacetronix Ltd. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Kuwait cardiovascular devices market appears promising, driven by ongoing technological advancements and increased healthcare investments. As the government continues to prioritize healthcare infrastructure, the integration of telemedicine and AI technologies is expected to enhance patient monitoring and treatment efficiency. Additionally, the growing geriatric population will further fuel demand for innovative cardiovascular solutions, ensuring that healthcare providers can meet the evolving needs of patients in future.
| Segment | Sub-Segments |
|---|---|
| By Type | Coronary Stents Pacemakers Implantable Cardioverter Defibrillators (ICDs) Cardiac Catheters Heart Valves (Mechanical & Tissue) Cardiac Rhythm Management Devices (including CRT, Holter Monitors) Diagnostic Devices (ECG, Echocardiography, Cardiac MRI, CT) Monitoring Devices (Wearable & Remote Monitors) Others (e.g., Vascular Grafts, Balloons) |
| By End-User | Hospitals Cardiac Clinics Ambulatory Surgical Centers Home Healthcare Research & Academic Institutions Others |
| By Distribution Channel | Direct Sales Distributors/Local Agents Online Sales Others |
| By Application | Coronary Artery Disease Arrhythmia Management Heart Failure Structural Heart Disease Diagnostic & Monitoring Others |
| By Technology | Invasive Technology Non-Invasive Technology Minimally Invasive Technology Hybrid Technology Others |
| By Patient Demographics | Pediatric Patients Adult Patients Geriatric Patients Others |
| By Policy Support | Government Subsidies Tax Incentives Research Grants Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Cardiovascular Device Usage in Hospitals | 100 | Cardiologists, Hospital Administrators |
| Market Insights from Medical Device Distributors | 60 | Sales Managers, Distribution Executives |
| Patient Perspectives on Cardiovascular Treatments | 50 | Patients with cardiovascular conditions, Caregivers |
| Regulatory Insights from Health Authorities | 40 | Regulatory Affairs Specialists, Health Policy Makers |
| Trends in Cardiovascular Device Innovation | 45 | R&D Managers, Product Development Leads |
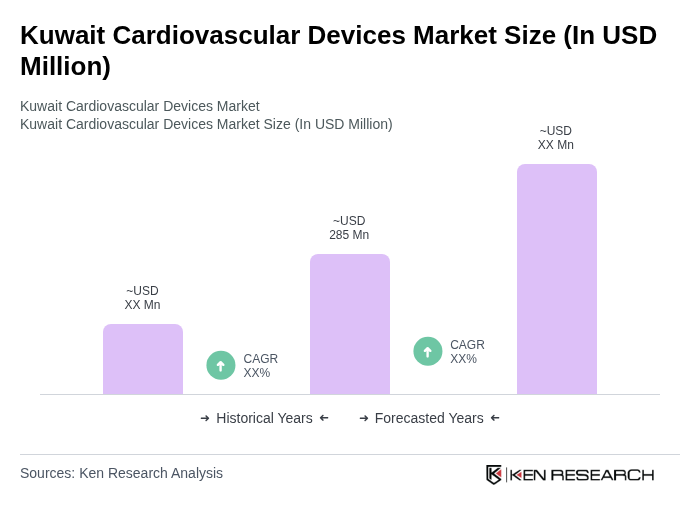
The Kuwait Cardiovascular Devices Market is valued at approximately USD 285 million, reflecting a significant growth driven by the increasing prevalence of cardiovascular diseases and advancements in medical technology.