Region:Middle East
Author(s):Shubham
Product Code:KRAC4299
Pages:85
Published On:October 2025
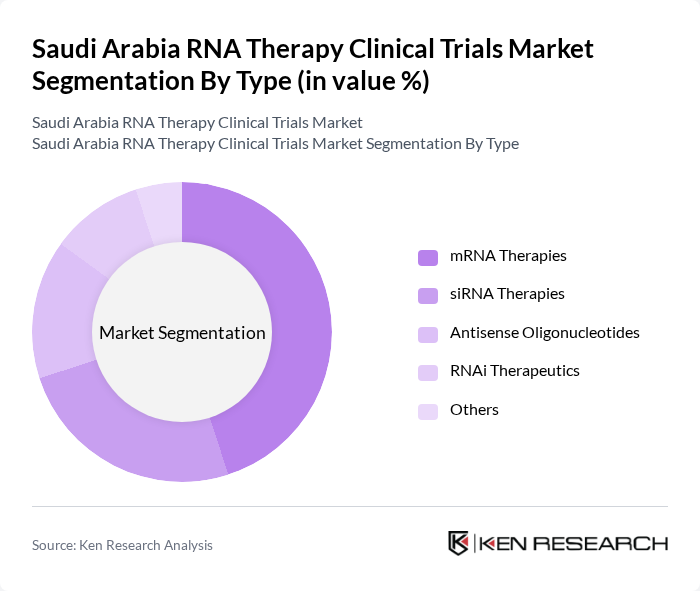
By Type:The RNA therapy clinical trials market can be segmented into various types, including mRNA therapies, siRNA therapies, antisense oligonucleotides, RNAi therapeutics, and others. Among these, mRNA therapies are gaining significant traction due to their role in vaccine development and potential applications in treating various diseases. The increasing focus on personalized medicine and the rapid advancements in mRNA technology are driving the growth of this segment.
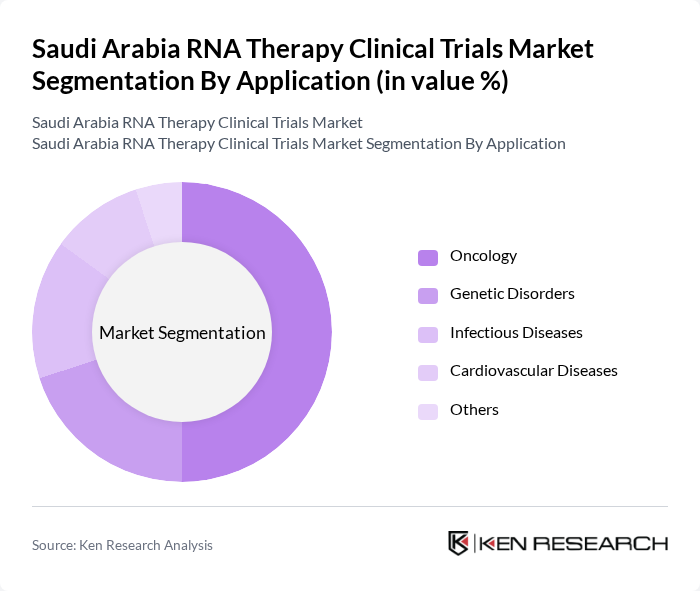
By Application:The applications of RNA therapies in clinical trials include oncology, genetic disorders, infectious diseases, cardiovascular diseases, and others. Oncology is the leading application area, driven by the increasing incidence of cancer and the need for innovative treatment options. The ability of RNA therapies to target specific cancer cells and enhance the efficacy of existing treatments is propelling this segment's growth. Rare genetic and hereditary diseases are also a fast-growing segment due to the precision and promise of RNA-based interventions.
The Saudi Arabia RNA Therapy Clinical Trials Market is characterized by a dynamic mix of regional and international players. Leading participants such as Moderna, Inc., BioNTech SE, Alnylam Pharmaceuticals, Inc., Ionis Pharmaceuticals, Inc., Sarepta Therapeutics, Inc., CureVac N.V., Arcturus Therapeutics Ltd., Pfizer Inc., Novartis AG, GSK plc, Sanofi S.A., IQVIA Holdings Inc., ICON plc, Labcorp Drug Development (formerly Covance), King Saud University (Research Collaborator), King Abdulaziz University (Research Collaborator), Saudi Clinical Trials Expert (SCTE) Network, Saudi Food and Drug Authority (SFDA) (Regulatory Body) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the RNA therapy clinical trials market in Saudi Arabia appears promising, driven by increasing investments in healthcare infrastructure and a growing emphasis on precision medicine. As the government continues to support biotech initiatives, the landscape for RNA therapies is expected to evolve significantly. Enhanced collaboration with international research institutions will likely accelerate innovation, while the integration of advanced technologies, such as artificial intelligence, will streamline trial processes and improve patient outcomes.
| Segment | Sub-Segments |
|---|---|
| By Type | mRNA Therapies siRNA Therapies Antisense Oligonucleotides RNAi Therapeutics Others |
| By Application | Oncology Genetic Disorders Infectious Diseases Cardiovascular Diseases Others |
| By End-User | Hospitals Research Institutions Pharmaceutical Companies Contract Research Organizations Others |
| By Funding Source | Government Grants Private Investments Venture Capital Public-Private Partnerships Others |
| By Clinical Phase | Phase I Trials Phase II Trials Phase III Trials Phase IV Trials Others |
| By Region | Central Region Eastern Region Western Region Southern Region Others |
| By Duration | Short-term Trials Long-term Trials Ongoing Trials Completed Trials Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Oncology RNA Therapy Trials | 60 | Oncologists, Clinical Trial Managers |
| Genetic Disorder RNA Treatments | 50 | Pediatricians, Genetic Counselors |
| Infectious Disease RNA Research | 40 | Infectious Disease Specialists, Research Scientists |
| Regulatory Perspectives on RNA Therapies | 40 | Regulatory Affairs Managers, Compliance Officers |
| Biotech Industry Insights | 50 | Biotech Executives, Market Analysts |
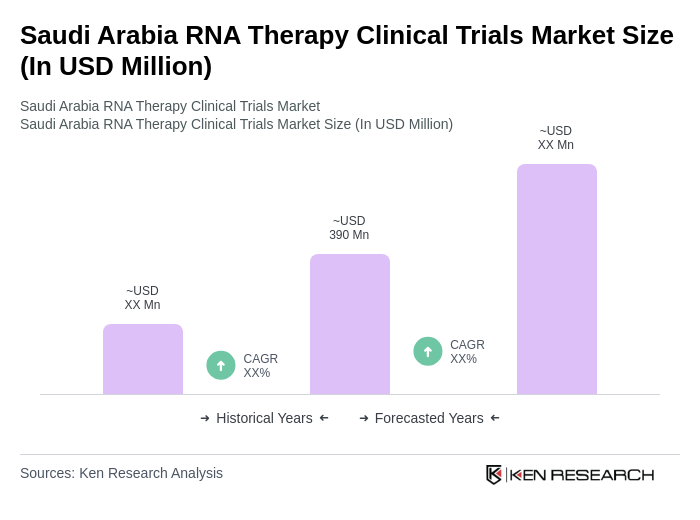
The Saudi Arabia RNA Therapy Clinical Trials Market is valued at approximately USD 390 million, reflecting significant growth driven by advancements in biotechnology, increased R&D investment, and a rising prevalence of genetic disorders and cancers.