Region:Middle East
Author(s):Dev
Product Code:KRAA8363
Pages:84
Published On:November 2025
By Therapy Type:
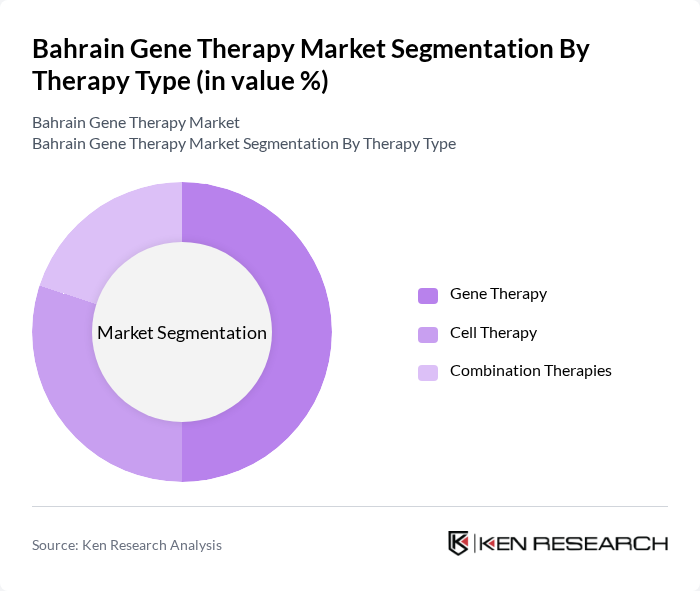
The therapy type segmentation includes Gene Therapy, Cell Therapy, and Combination Therapies. Among these, Gene Therapy is the fastest-growing segment, driven by its potential to treat previously incurable genetic disorders. The increasing number of clinical trials and successful product launches in this area has led to heightened interest from both healthcare providers and patients. Cell Therapy is also gaining traction, particularly in oncology, while Combination Therapies are being explored for their synergistic effects in treatment protocols. The market is witnessing a shift toward decentralized manufacturing and next-generation viral/non-viral delivery systems, supporting the growth of combination therapies .
By Therapeutic Area:
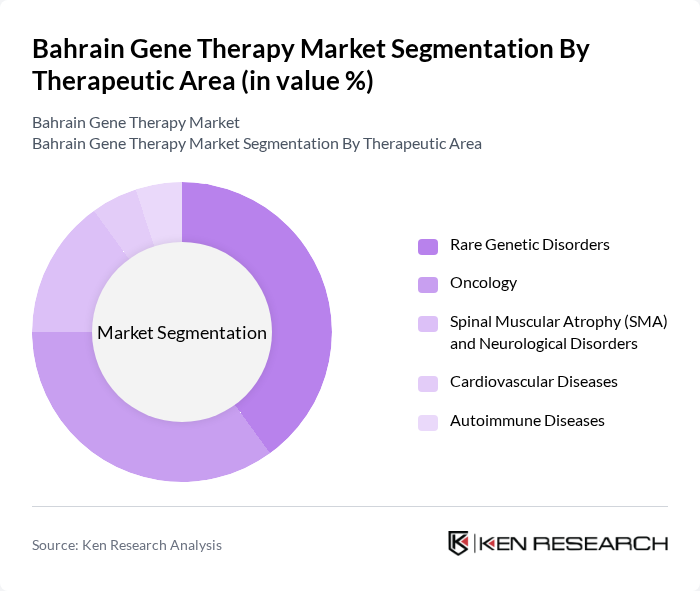
This segmentation includes Rare Genetic Disorders, Oncology, Spinal Muscular Atrophy (SMA) and Neurological Disorders, Cardiovascular Diseases, and Autoimmune Diseases. Rare Genetic Disorders represent the highest growth area, fueled by increasing awareness and diagnosis rates. Oncology, particularly CAR-T cell therapies, is also a significant focus due to the rising incidence of cancer and the introduction of new cell therapy assets. The other therapeutic areas are gradually gaining attention as research progresses, but they currently lag behind in terms of market share. The GCC region is noted for its focus on rare diseases and cancer, with clinical-scale manufacturing leading market activity .
The Bahrain Gene Therapy Market is characterized by a dynamic mix of regional and international players. Leading participants such as Novartis AG, Gilead Sciences, Inc., Spark Therapeutics, Inc., Bluebird Bio, Inc., Amgen Inc., Regeneron Pharmaceuticals, Inc., CRISPR Therapeutics AG, Editas Medicine, Inc., Intellia Therapeutics, Inc., Sarepta Therapeutics, Inc., Roche Holding AG, Pfizer Inc., Johnson & Johnson, Celgene Corporation, AbbVie Inc., Avernus Pharma, Opal Biopharma contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Bahrain gene therapy market appears promising, driven by ongoing advancements in biotechnology and increasing collaboration between local and international firms. As the government continues to support healthcare innovation through favorable policies, the market is expected to witness a surge in clinical trials and research initiatives. Additionally, the growing focus on personalized medicine will likely enhance treatment options, catering to the unique genetic profiles of patients, thereby improving overall healthcare outcomes in the region.
| Segment | Sub-Segments |
|---|---|
| By Therapy Type | Gene Therapy (fastest growing segment at highest CAGR) Cell Therapy Combination Therapies |
| By Therapeutic Area | Rare Genetic Disorders (highest growth CAGR) Oncology (including CAR-T cell therapies for blood cancers) Spinal Muscular Atrophy (SMA) and Neurological Disorders Cardiovascular Diseases Autoimmune Diseases |
| By Vector/Delivery Type | Viral Vector Therapies (adeno-associated viruses, lentiviruses) Non-Viral Vector Therapies CRISPR-based Gene Editing Antisense Oligonucleotides |
| By Manufacturing Scale & Service | Clinical-Scale Manufacturing (63% market share in 2024) Commercial-Scale Manufacturing Contract Manufacturing Services |
| By End-User | Biopharma & Biotechnology Companies (42% market share in 2024) Hospitals and Healthcare Providers Research Institutions and Academic Centers Contract Research Organizations (CROs) |
| By Patient Demographics | Pediatric Patients (including children over 12 for thalassemia and sickle cell anemia treatment) Adult Patients Geriatric Patients |
| By Geographic Region | Central Bahrain (Manama) Northern Governorate Southern Governorate Muharraq Governorate |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Oncology Gene Therapy Programs | 100 | Oncologists, Clinical Researchers |
| Rare Disease Treatment Initiatives | 60 | Geneticists, Rare Disease Specialists |
| Healthcare Policy Makers | 40 | Health Ministry Officials, Policy Analysts |
| Patient Advocacy Groups | 40 | Patient Representatives, Advocacy Leaders |
| Clinical Trial Coordinators | 50 | Clinical Research Coordinators, Trial Managers |
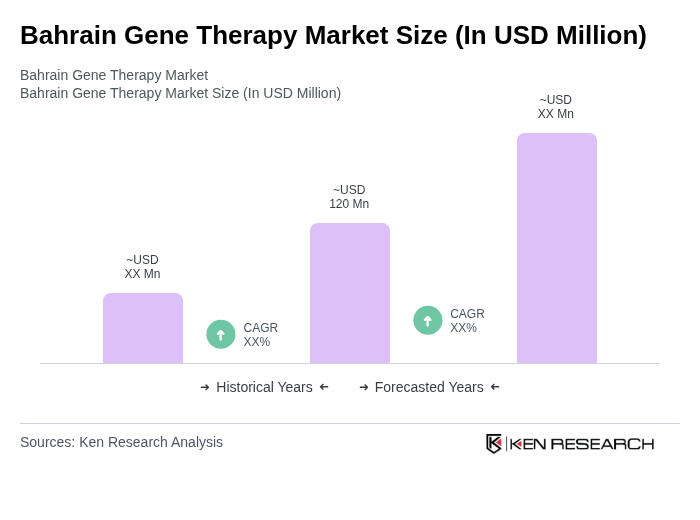
The Bahrain Gene Therapy Market is valued at approximately USD 120 million, reflecting significant growth driven by advancements in biotechnology, increasing prevalence of genetic disorders, and rising investments in research and development within the region.