Region:Middle East
Author(s):Shubham
Product Code:KRAB7841
Pages:91
Published On:October 2025
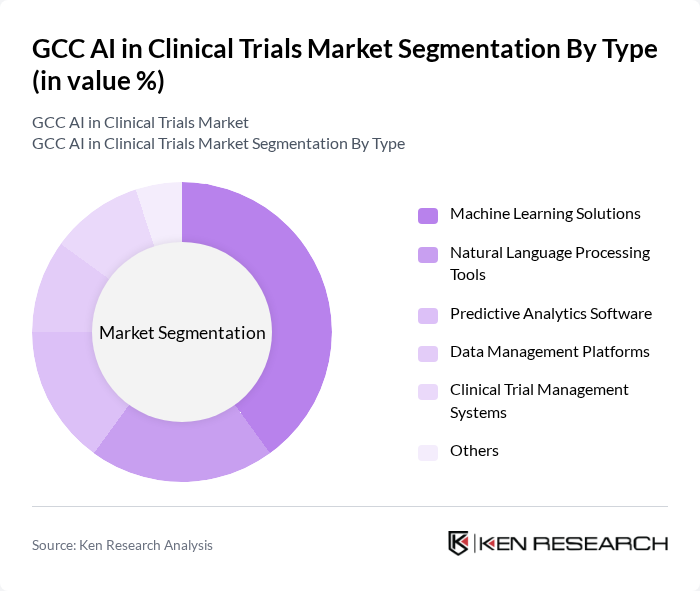
By Type:The market is segmented into various types of AI solutions utilized in clinical trials. The subsegments include Machine Learning Solutions, Natural Language Processing Tools, Predictive Analytics Software, Data Management Platforms, Clinical Trial Management Systems, and Others. Among these, Machine Learning Solutions are leading the market due to their ability to analyze vast datasets and improve decision-making processes in clinical trials. The increasing complexity of clinical data and the need for real-time insights are driving the adoption of machine learning technologies.
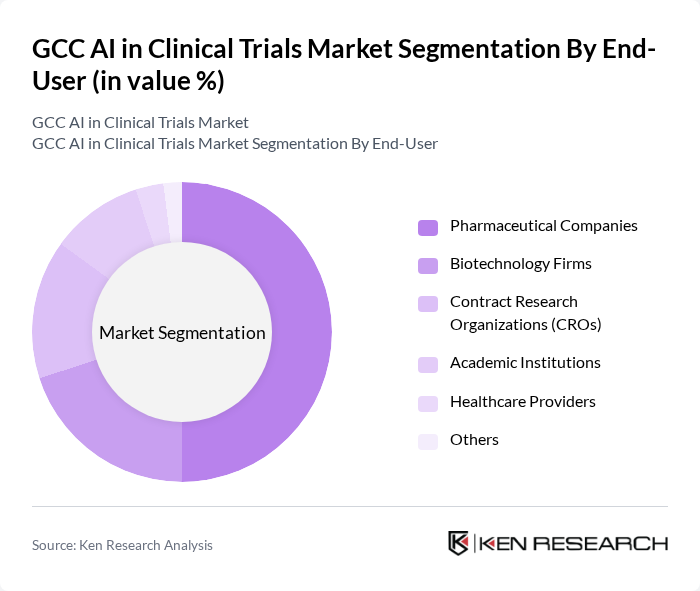
By End-User:The end-user segmentation includes Pharmaceutical Companies, Biotechnology Firms, Contract Research Organizations (CROs), Academic Institutions, Healthcare Providers, and Others. Pharmaceutical Companies are the dominant end-users in the market, leveraging AI technologies to enhance drug development processes and streamline clinical trials. The increasing pressure to reduce time-to-market for new drugs and the need for cost-effective solutions are driving pharmaceutical companies to adopt AI in their clinical trial operations.
The GCC AI in Clinical Trials Market is characterized by a dynamic mix of regional and international players. Leading participants such as IBM Watson Health, Oracle Corporation, Medidata Solutions, Parexel International Corporation, Covance Inc., Syneos Health, CRF Health, Veeva Systems Inc., BioClinica, PPD Inc., ERT, ICON plc, Charles River Laboratories, WuXi AppTec, Medpace Holdings, Inc. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the GCC AI in clinical trials market appears promising, driven by technological advancements and increasing regulatory support. As healthcare providers and pharmaceutical companies embrace AI, the integration of real-world evidence and predictive analytics will enhance trial efficiency. Furthermore, the shift towards decentralized clinical trials is expected to improve patient engagement and data collection, ultimately leading to faster drug development cycles and better patient outcomes in the region.
| Segment | Sub-Segments |
|---|---|
| By Type | Machine Learning Solutions Natural Language Processing Tools Predictive Analytics Software Data Management Platforms Clinical Trial Management Systems Others |
| By End-User | Pharmaceutical Companies Biotechnology Firms Contract Research Organizations (CROs) Academic Institutions Healthcare Providers Others |
| By Application | Patient Recruitment and Retention Data Analysis and Management Risk Assessment and Monitoring Regulatory Compliance Others |
| By Region | Saudi Arabia United Arab Emirates Qatar Kuwait Oman Bahrain Others |
| By Investment Source | Private Equity Venture Capital Government Funding Corporate Investments Others |
| By Policy Support | Research Grants Tax Incentives Subsidies for Technology Adoption Regulatory Frameworks Others |
| By Clinical Trial Phase | Phase I Phase II Phase III Phase IV Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Pharmaceutical Companies | 100 | Clinical Research Directors, Regulatory Affairs Managers |
| Biotechnology Firms | 80 | R&D Managers, Data Scientists |
| Healthcare Institutions | 70 | Clinical Trial Coordinators, Ethics Committee Members |
| AI Technology Providers | 60 | Product Managers, Technical Leads |
| Regulatory Bodies | 50 | Policy Makers, Compliance Officers |
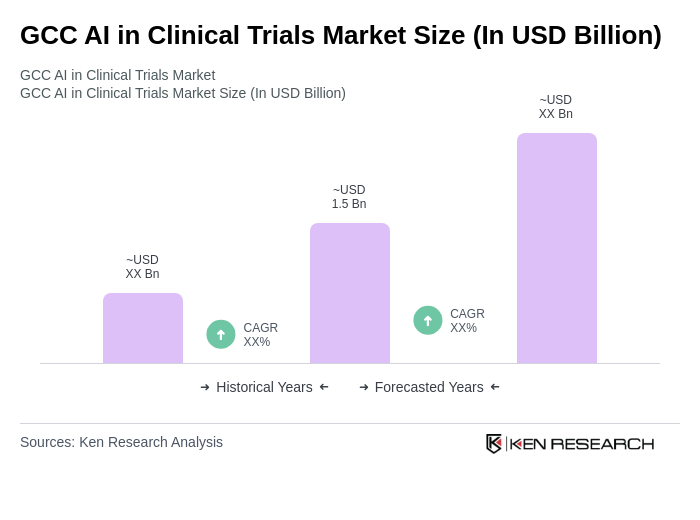
The GCC AI in Clinical Trials Market is valued at approximately USD 1.5 billion, reflecting significant growth driven by the increasing adoption of AI technologies in healthcare, aimed at enhancing clinical trial efficiency and accuracy.