Region:Middle East
Author(s):Shubham
Product Code:KRAD2480
Pages:86
Published On:January 2026
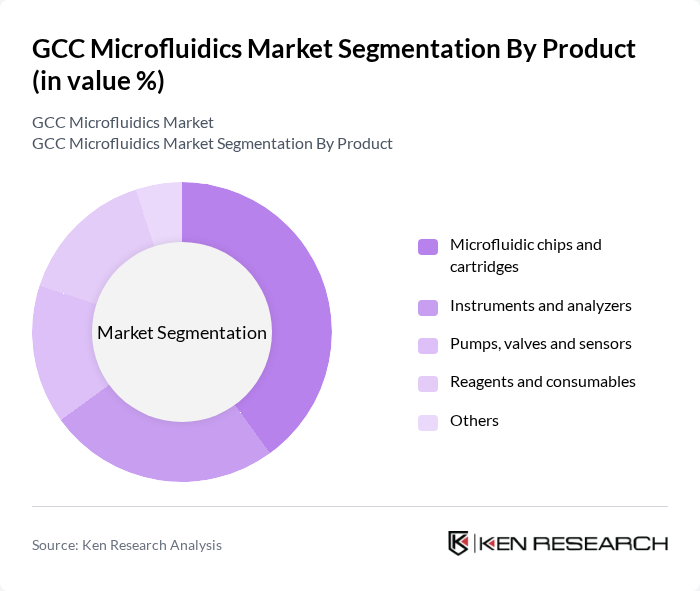
By Product:The product segmentation of the market includes microfluidic chips and cartridges, instruments and analyzers, pumps, valves and sensors, reagents and consumables, and others. Among these, microfluidic chips and cartridges are leading the market due to their essential role in diagnostics and point-of-care testing. The increasing demand for rapid and accurate testing solutions in healthcare drives the growth of this subsegment, as healthcare providers seek to enhance patient outcomes through efficient diagnostic tools.
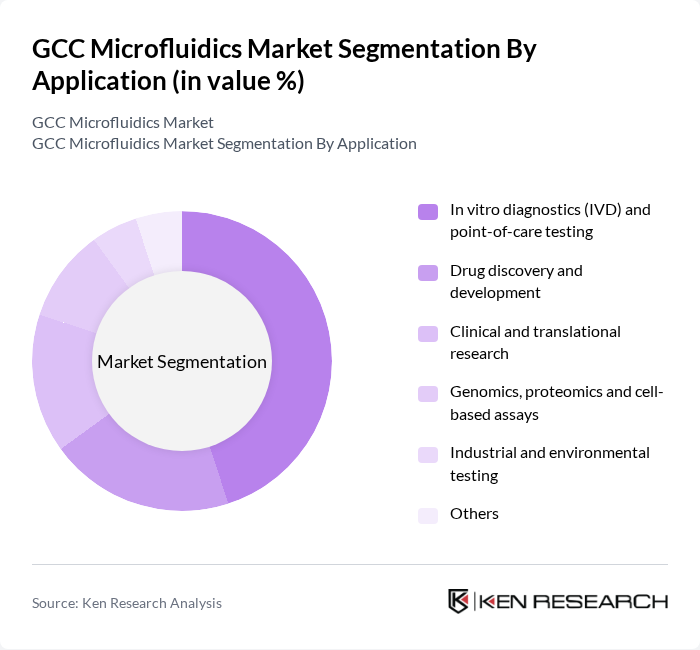
By Application:The application segmentation includes in vitro diagnostics (IVD) and point-of-care testing, drug discovery and development, clinical and translational research, genomics, proteomics and cell-based assays, industrial and environmental testing, and others. The in vitro diagnostics and point-of-care testing segment is the most significant, driven by the increasing need for rapid diagnostic solutions in clinical settings. The growing prevalence of infectious diseases and the demand for personalized medicine further bolster this segment's growth, as healthcare providers prioritize timely and accurate testing.
The GCC Microfluidics Market is characterized by a dynamic mix of regional and international players. Leading participants such as Thermo Fisher Scientific Inc., Agilent Technologies Inc., Bio-Rad Laboratories Inc., Revvity Inc. (PerkinElmer), Illumina Inc., Merck KGaA (MilliporeSigma), F. Hoffmann-La Roche Ltd, Becton, Dickinson and Company, QIAGEN N.V., 10x Genomics Inc., Micronit Microtechnologies B.V. (Micronit Microfluidics), Cellix Ltd, Elveflow (Elvesys Group), Blacktrace Holdings Ltd, Dolomite Microfluidics contribute to innovation, geographic expansion, and service delivery in this space.
The future of the microfluidics market in the GCC appears promising, driven by technological advancements and increasing healthcare demands. As the region invests in healthcare infrastructure, the integration of AI and machine learning into microfluidic devices is expected to enhance diagnostic accuracy and efficiency. Furthermore, the shift towards personalized medicine will likely create new avenues for growth, as tailored healthcare solutions become more prevalent in the region's medical landscape.
| Segment | Sub-Segments |
|---|---|
| By Product | Microfluidic chips and cartridges Instruments and analyzers Pumps, valves and sensors Reagents and consumables Others |
| By Application | In vitro diagnostics (IVD) and point-of-care testing Drug discovery and development Clinical and translational research Genomics, proteomics and cell-based assays Industrial and environmental testing Others |
| By End-User | Hospitals and diagnostic centers Pharmaceutical and biotechnology companies Academic and research institutions Contract research organizations (CROs) Others |
| By Material | Polymers (including PDMS) Glass Silicon Others |
| By Technology | Lab-on-a-chip Organ-on-chip Continuous flow microfluidics Droplet-based microfluidics Others |
| By Country | Saudi Arabia United Arab Emirates Qatar Kuwait Oman Bahrain |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Healthcare Diagnostics | 120 | Laboratory Managers, Clinical Researchers |
| Drug Delivery Systems | 90 | Pharmaceutical R&D Directors, Product Managers |
| Biotechnology Research Applications | 80 | Biotech Researchers, Lab Technicians |
| Microfluidics Device Manufacturing | 70 | Manufacturing Engineers, Quality Assurance Managers |
| Regulatory Compliance in Microfluidics | 60 | Regulatory Affairs Specialists, Compliance Officers |
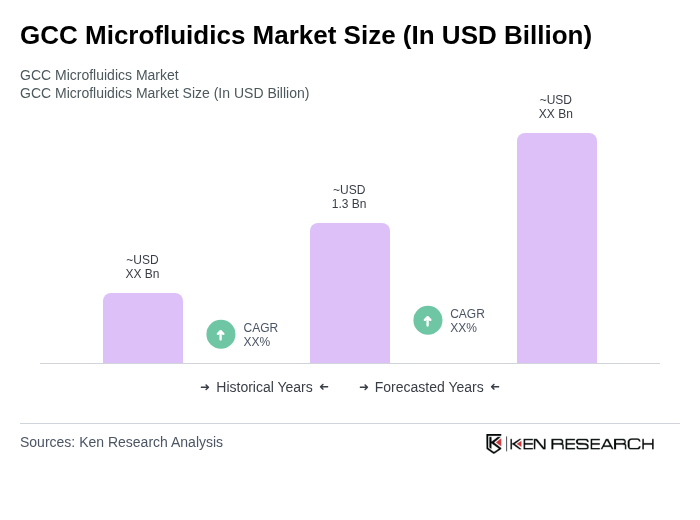
The GCC Microfluidics Market is valued at approximately USD 1.3 billion, driven by advancements in healthcare technology, increasing demand for point-of-care testing, and the rising prevalence of chronic diseases in the region.