Region:Middle East
Author(s):Dev
Product Code:KRAC3568
Pages:81
Published On:January 2026
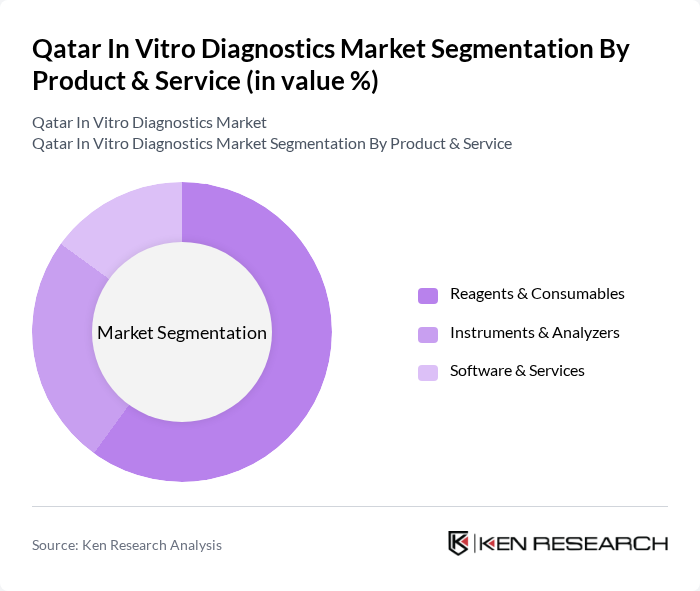
By Product & Service:The product and service segment of the Qatar In Vitro Diagnostics Market includes various subsegments such as reagents & consumables, instruments & analyzers, and software & services. Among these, reagents & consumables dominate the market due to their essential role in routine and specialized diagnostic testing processes and their recurring nature in every test performed. The increasing number of diagnostic tests performed in hospitals, centralized laboratories, and reference labs for chronic disease monitoring, infectious disease screening, and preventive check?ups drives the demand for these products, making them a critical component of the overall market.
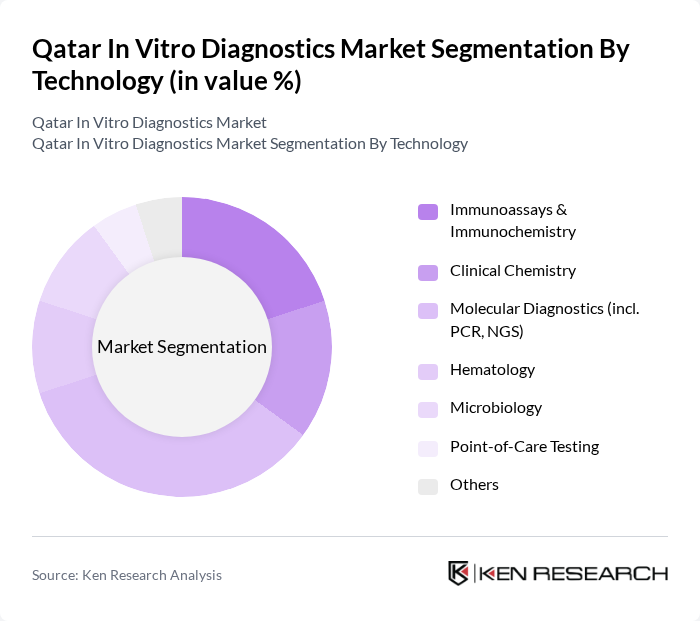
By Technology:The technology segment encompasses various diagnostic methods, including immunoassays & immunochemistry, clinical chemistry, molecular diagnostics (including PCR and NGS), hematology, microbiology, point-of-care testing, and others. Molecular diagnostics has been expanding rapidly due to its ability to provide rapid and accurate results, particularly in infectious disease testing, oncology, and genetic analysis, and is now among the key growth drivers within the technology mix. The growing trend towards personalized medicine, wider use of PCR?based and next?generation sequencing platforms, and the need for precise diagnostic tools to guide targeted therapies further enhance the demand for this technology in Qatar and the broader Middle East.
The Qatar In Vitro Diagnostics Market is characterized by a dynamic mix of regional and international players. Leading participants such as Siemens Healthineers, Roche Diagnostics, Abbott Laboratories, Thermo Fisher Scientific, Bio-Rad Laboratories, Beckman Coulter (a Danaher company), Ortho Clinical Diagnostics (QuidelOrtho Corporation), QIAGEN N.V., Hologic Inc., Agilent Technologies Inc., Revvity, Inc. (formerly PerkinElmer’s applied, food and enterprise services), Becton, Dickinson and Company (BD), Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Sysmex Corporation, DiaSorin S.p.A. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the in vitro diagnostics market in Qatar appears promising, driven by ongoing advancements in technology and increasing healthcare investments. The integration of artificial intelligence in diagnostic processes is expected to enhance accuracy and efficiency, while the expansion of telemedicine will facilitate remote diagnostics. As the healthcare landscape evolves, stakeholders must adapt to these trends to capitalize on emerging opportunities and improve patient care outcomes in the region.
| Segment | Sub-Segments |
|---|---|
| By Product & Service | Reagents & Consumables Instruments & Analyzers Software & Services |
| By Technology | Immunoassays & Immunochemistry Clinical Chemistry Molecular Diagnostics (incl. PCR, NGS) Hematology Microbiology Point-of-Care Testing Others |
| By Application | Infectious Diseases Diabetes Cancer / Oncology Cardiology Autoimmune & Inflammatory Diseases Nephrology & Kidney Disorders Genetic & Prenatal Testing Others |
| By End-User | Hospitals Diagnostic & Reference Laboratories Clinics & Physician Office Labs Academic & Research Institutions Home Care & Self-testing Others |
| By Testing Location | Centralized Laboratory Testing Point-of-Care / Near-Patient Testing Home-based Testing |
| By Distribution Channel | Direct Sales Local Distributors & Importers Group Purchasing Organizations (GPOs) & Tenders Online & E-Procurement Portals Others |
| By Region | Doha Al Rayyan Umm Salal Al Wakrah Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Clinical Laboratories | 90 | Laboratory Managers, Medical Technologists |
| Hospitals and Healthcare Facilities | 70 | Healthcare Administrators, Pathology Heads |
| Diagnostic Equipment Suppliers | 60 | Sales Managers, Product Specialists |
| Regulatory Bodies | 50 | Regulatory Affairs Officers, Compliance Managers |
| Research Institutions | 40 | Research Scientists, Clinical Researchers |
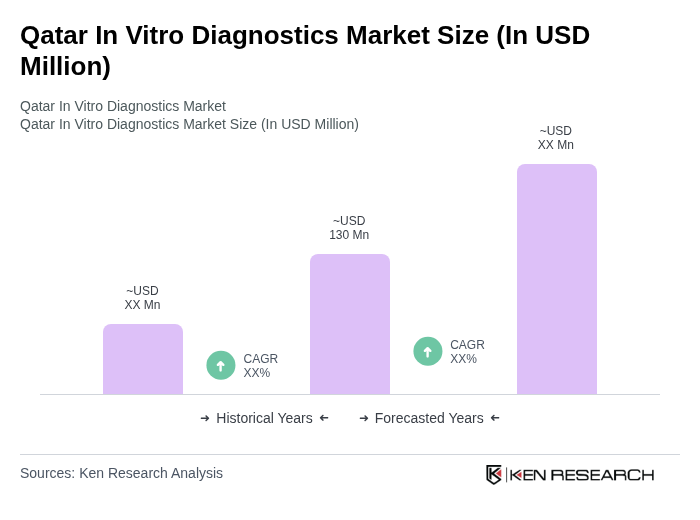
The Qatar In Vitro Diagnostics Market is valued at approximately USD 130 million, reflecting a significant growth driven by the increasing prevalence of chronic diseases, advancements in diagnostic technologies, and a focus on preventive healthcare.