Region:Middle East
Author(s):Rebecca
Product Code:KRAB7757
Pages:90
Published On:October 2025
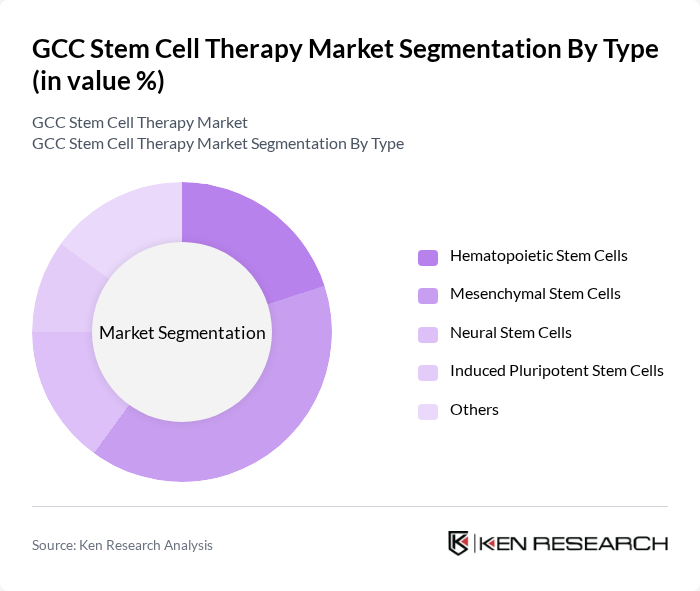
By Type:The market is segmented into various types of stem cells, including Hematopoietic Stem Cells, Mesenchymal Stem Cells, Neural Stem Cells, Induced Pluripotent Stem Cells, and Others. Among these, Mesenchymal Stem Cells are currently leading the market due to their versatility in treating a wide range of conditions, including orthopedic and cardiovascular diseases. Their ability to differentiate into various cell types and their immunomodulatory properties make them highly sought after in clinical applications. The increasing number of clinical trials and research studies focusing on Mesenchymal Stem Cells further solidifies their dominance in the market.
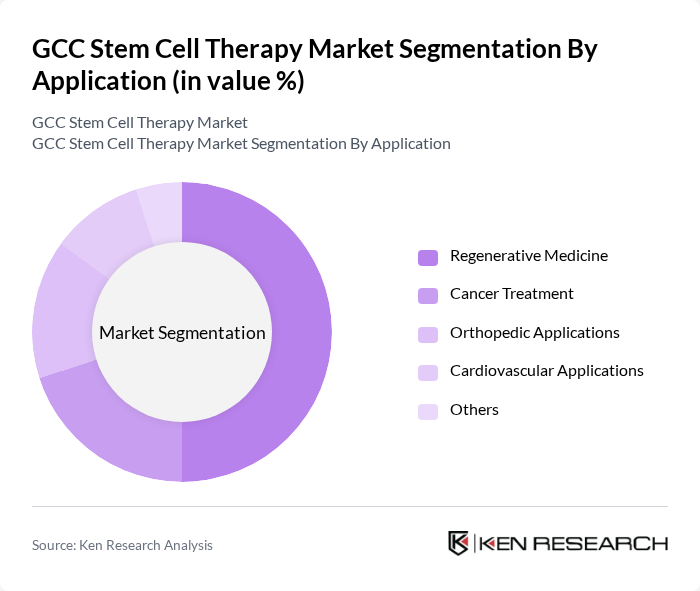
By Application:The applications of stem cell therapy include Regenerative Medicine, Cancer Treatment, Orthopedic Applications, Cardiovascular Applications, and Others. Regenerative Medicine is the leading application area, driven by the increasing demand for innovative treatments for degenerative diseases and injuries. The growing focus on personalized medicine and the ability of stem cells to regenerate damaged tissues contribute to the rising adoption of regenerative therapies. Additionally, advancements in research and technology are expanding the scope of applications, further enhancing the market's growth.
The GCC Stem Cell Therapy Market is characterized by a dynamic mix of regional and international players. Leading participants such as Celltex Therapeutics Corporation, Osiris Therapeutics, Inc., Athersys, Inc., Mesoblast Limited, StemCells, Inc., TiGenix N.V., Pluristem Therapeutics Inc., Cellerant Therapeutics, Inc., Lonza Group AG, Cryo-Cell International, Inc., ReNeuron Group plc, Gamida Cell Ltd., AlloVir, Inc., Vericel Corporation, Fate Therapeutics, Inc. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the GCC stem cell therapy market appears promising, driven by ongoing advancements in technology and increasing public acceptance. As healthcare systems evolve, the integration of personalized medicine and innovative treatment modalities will likely enhance patient outcomes. Furthermore, the collaboration between governments and private sectors to establish robust regulatory frameworks will facilitate research and clinical trials, ensuring that stem cell therapies become more widely available and accepted in mainstream healthcare practices.
| Segment | Sub-Segments |
|---|---|
| By Type | Hematopoietic Stem Cells Mesenchymal Stem Cells Neural Stem Cells Induced Pluripotent Stem Cells Others |
| By Application | Regenerative Medicine Cancer Treatment Orthopedic Applications Cardiovascular Applications Others |
| By End-User | Hospitals Research Institutions Biopharmaceutical Companies Others |
| By Source | Adult Stem Cells Embryonic Stem Cells Cord Blood Stem Cells Others |
| By Treatment Type | Autologous Transplantation Allogeneic Transplantation Others |
| By Distribution Channel | Direct Sales Online Sales Distributors Others |
| By Pricing Model | Premium Pricing Competitive Pricing Value-Based Pricing Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Hematopoietic Stem Cell Therapy | 100 | Hematologists, Oncologists |
| Mesenchymal Stem Cell Therapy | 80 | Orthopedic Surgeons, Regenerative Medicine Specialists |
| Clinical Trials and Research | 60 | Clinical Researchers, Trial Coordinators |
| Patient Experience and Outcomes | 75 | Patients, Caregivers |
| Healthcare Policy and Regulation | 50 | Health Policy Analysts, Regulatory Affairs Managers |
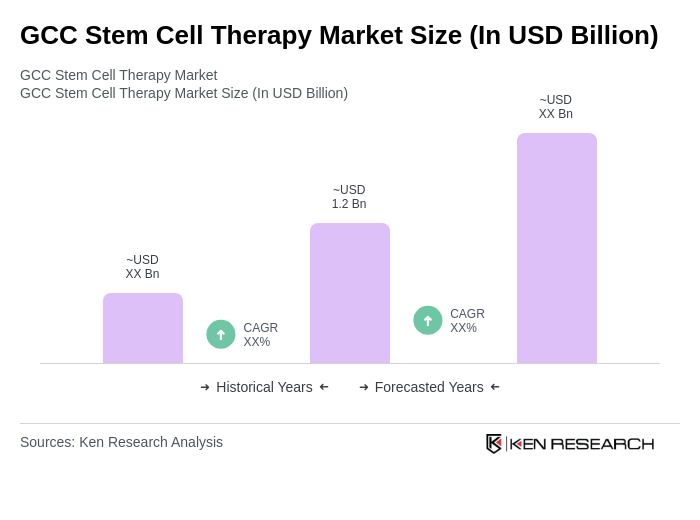
The GCC Stem Cell Therapy Market is valued at approximately USD 1.2 billion, reflecting significant growth driven by advancements in medical technology, increasing chronic disease prevalence, and rising investments in regenerative medicine across the region.