Region:Asia
Author(s):Geetanshi
Product Code:KRAD5869
Pages:80
Published On:December 2025
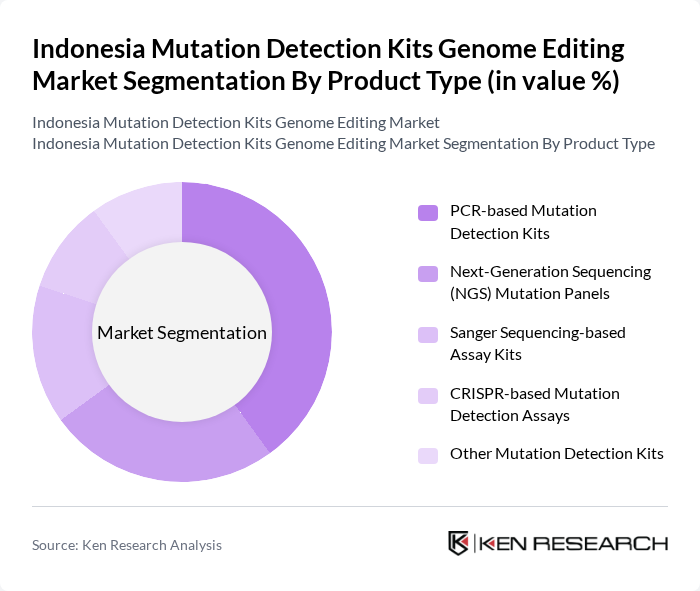
By Product Type:The product type segmentation includes various kits utilized for mutation detection, each catering to specific needs in genomic research and diagnostics. The subsegments include PCR-based Mutation Detection Kits, Next-Generation Sequencing (NGS) Mutation Panels, Sanger Sequencing-based Assay Kits, CRISPR-based Mutation Detection Assays, and Other Mutation Detection Kits. Among these, PCR-based Mutation Detection Kits are leading the market due to their widespread application in clinical diagnostics and research settings, offering high sensitivity and specificity for detecting genetic mutations.
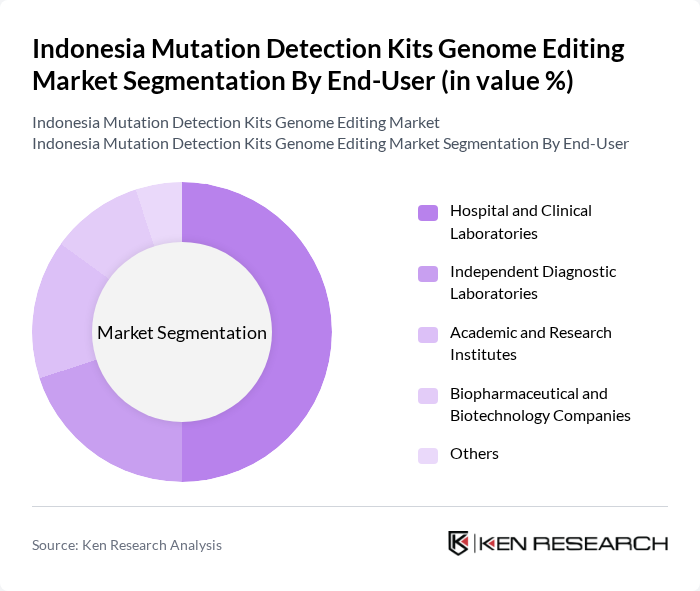
By End-User:The end-user segmentation encompasses various entities utilizing mutation detection kits, including Hospital and Clinical Laboratories, Independent Diagnostic Laboratories, Academic and Research Institutes, Biopharmaceutical and Biotechnology Companies, and Others. Hospital and Clinical Laboratories dominate this segment, driven by the increasing demand for accurate diagnostic tools and the growing prevalence of genetic disorders, which necessitate reliable mutation detection for effective treatment planning.
The Indonesia Mutation Detection Kits Genome Editing Market is characterized by a dynamic mix of regional and international players. Leading participants such as Thermo Fisher Scientific Inc., Illumina, Inc., Agilent Technologies, Inc., Bio-Rad Laboratories, Inc., QIAGEN N.V., F. Hoffmann-La Roche Ltd (Roche Diagnostics), Merck KGaA (MilliporeSigma), New England Biolabs, Inc., Takara Bio Inc., Integrated DNA Technologies, Inc. (IDT), BGI Genomics Co., Ltd., Synthego Corporation, PT KalGen DNA, PT Interskala Medika Indonesia, PT Jayamas Medica Industri Tbk contribute to innovation, geographic expansion, and service delivery in this space.
The future of the mutation detection kits market in Indonesia appears promising, driven by technological advancements and increasing healthcare investments. As the government prioritizes biotechnology, the integration of AI in genetic analysis is expected to enhance diagnostic accuracy and efficiency. Furthermore, the expansion of telemedicine and remote diagnostics will facilitate access to mutation detection services, particularly in underserved regions. These trends indicate a robust growth trajectory for the market, fostering innovation and improving patient care in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Product Type | PCR-based Mutation Detection Kits Next-Generation Sequencing (NGS) Mutation Panels Sanger Sequencing-based Assay Kits CRISPR-based Mutation Detection Assays Other Mutation Detection Kits |
| By End-User | Hospital and Clinical Laboratories Independent Diagnostic Laboratories Academic and Research Institutes Biopharmaceutical and Biotechnology Companies Others |
| By Application | Oncology and Solid Tumor Mutation Testing Inherited and Rare Genetic Disorder Testing Infectious Disease and Viral Variant Detection Prenatal and Reproductive Genetic Testing Drug Development and Companion Diagnostics |
| By Distribution Channel | Direct Sales to End Users Regional Distributors and Importers Online Procurement Platforms Group Purchasing Organizations Others |
| By Region | Java Sumatra Bali and Nusa Tenggara Kalimantan Sulawesi, Maluku & Papua |
| By Genome Editing Technology | CRISPR/Cas Systems TALEN-based Genome Editing Zinc Finger Nuclease (ZFN) Technology Other Genome Editing Technologies |
| By Research Type | Academic and Translational Research Clinical Research and Trials Industrial and Contract Research Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Clinical Laboratories | 100 | Laboratory Managers, Geneticists |
| Research Institutions | 80 | Research Scientists, Biotech Researchers |
| Healthcare Providers | 70 | Medical Practitioners, Pathologists |
| Biotechnology Firms | 60 | Product Development Managers, Regulatory Affairs Specialists |
| Government Health Agencies | 50 | Policy Makers, Health Program Coordinators |
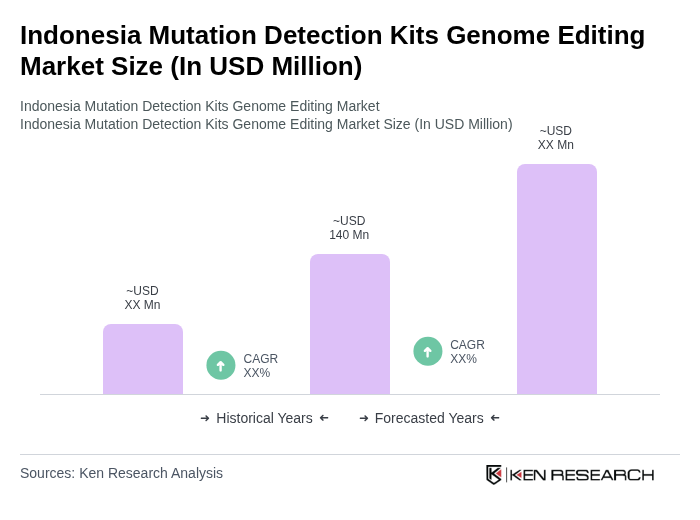
The Indonesia Mutation Detection Kits Genome Editing Market is valued at approximately USD 140 million, reflecting significant growth driven by advancements in genomic technologies and increasing demand for accurate diagnostic tools in healthcare.