Region:Middle East
Author(s):Rebecca
Product Code:KRAC9805
Pages:98
Published On:November 2025
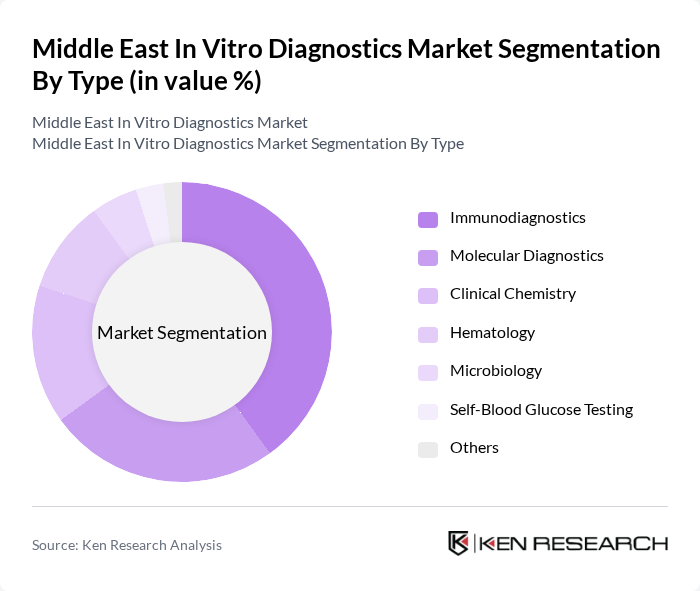
By Type:The market is segmented into Immunodiagnostics, Molecular Diagnostics, Clinical Chemistry, Hematology, Microbiology, Self-Blood Glucose Testing, and Others. Among these,Immunodiagnosticsis currently the leading subsegment, driven by the increasing demand for rapid and accurate diagnostic tests for infectious diseases and chronic conditions. The growing trend towards point-of-care testing, home diagnostics, and the integration of multiplex immunoassays has also contributed to the popularity of this segment. Molecular diagnostics is expanding rapidly due to technological innovations and the adoption of PCR and next-generation sequencing platforms for infectious disease and cancer screening .
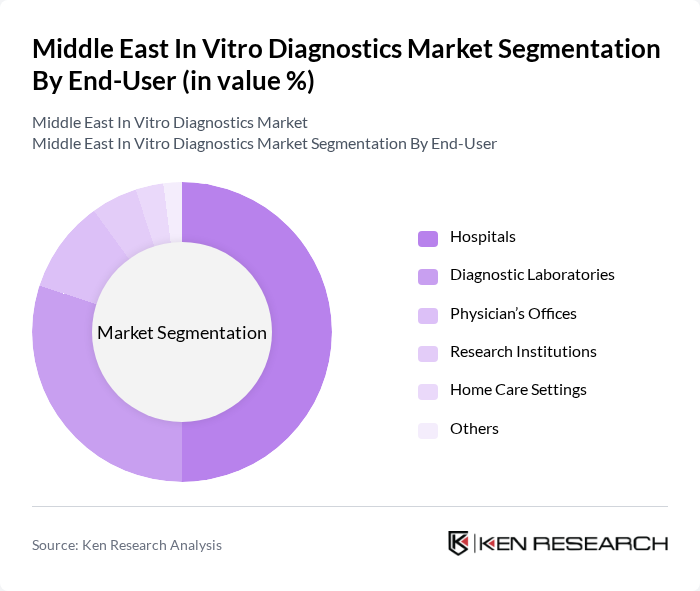
By End-User:The end-user segmentation includes Hospitals, Diagnostic Laboratories, Physician’s Offices, Research Institutions, Home Care Settings, and Others.Hospitalsare the dominant end-user segment, primarily due to the increasing number of patients requiring diagnostic tests and the growing trend of hospitals adopting advanced diagnostic technologies. The demand for comprehensive healthcare services in hospitals, especially for chronic and infectious disease management, has led to a significant rise in the utilization of in vitro diagnostic tests. Diagnostic laboratories are also experiencing growth, driven by the expansion of centralized laboratory networks and outsourcing of specialized testing .
The Middle East In Vitro Diagnostics Market is characterized by a dynamic mix of regional and international players. Leading participants such as Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, Thermo Fisher Scientific, Bio-Rad Laboratories, Becton, Dickinson and Company, QIAGEN, Hologic, Inc., Agilent Technologies, PerkinElmer, Inc., Danaher Corporation, Sysmex Corporation, Mindray Medical International Limited, Ortho Clinical Diagnostics, Grifols S.A., bioMérieux SA, Randox Laboratories Ltd., Quidel Corporation contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Middle East in vitro diagnostics market appears promising, driven by technological innovations and increasing healthcare investments. As the region continues to prioritize healthcare infrastructure, the integration of artificial intelligence and telemedicine is expected to enhance diagnostic accuracy and accessibility. Furthermore, the growing emphasis on personalized medicine will likely lead to tailored diagnostic solutions, improving patient care and outcomes. These trends indicate a dynamic evolution in the IVD landscape, fostering growth and development in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Type | Immunodiagnostics Molecular Diagnostics Clinical Chemistry Hematology Microbiology Self-Blood Glucose Testing Others |
| By End-User | Hospitals Diagnostic Laboratories Physician’s Offices Research Institutions Home Care Settings Others |
| By Application | Infectious Diseases Cancer Diagnostics Cardiovascular Diseases Diabetes Genetic Testing Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Others |
| By Region | GCC Countries (Saudi Arabia, UAE, Qatar, Kuwait, Oman, Bahrain) Levant Region (Jordan, Lebanon, Israel, Palestine, Syria) North Africa (Egypt, Morocco, Algeria, Tunisia, Libya) Others |
| By Technology | PCR Technology Next-Generation Sequencing Microarray Technology Immunochemistry Others |
| By Policy Support | Government Subsidies Tax Incentives Research Grants Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Clinical Laboratories | 100 | Laboratory Managers, Pathologists |
| Hospitals and Healthcare Facilities | 80 | Healthcare Administrators, Medical Directors |
| Diagnostic Equipment Manufacturers | 60 | Product Managers, Sales Directors |
| Regulatory Bodies | 40 | Regulatory Affairs Specialists, Compliance Officers |
| Research Institutions | 50 | Research Scientists, Clinical Researchers |
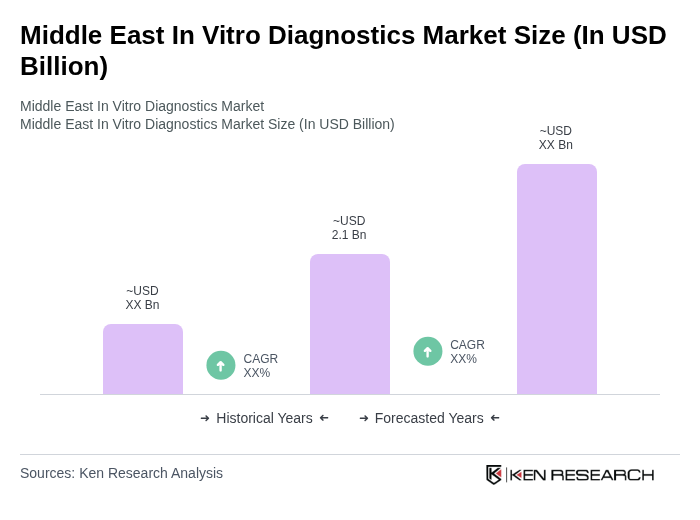
The Middle East In Vitro Diagnostics Market is valued at approximately USD 2.1 billion, driven by the rising prevalence of chronic diseases, advancements in diagnostic technologies, and a growing focus on preventive healthcare.