Region:Asia
Author(s):Rebecca
Product Code:KRAE3429
Pages:96
Published On:February 2026
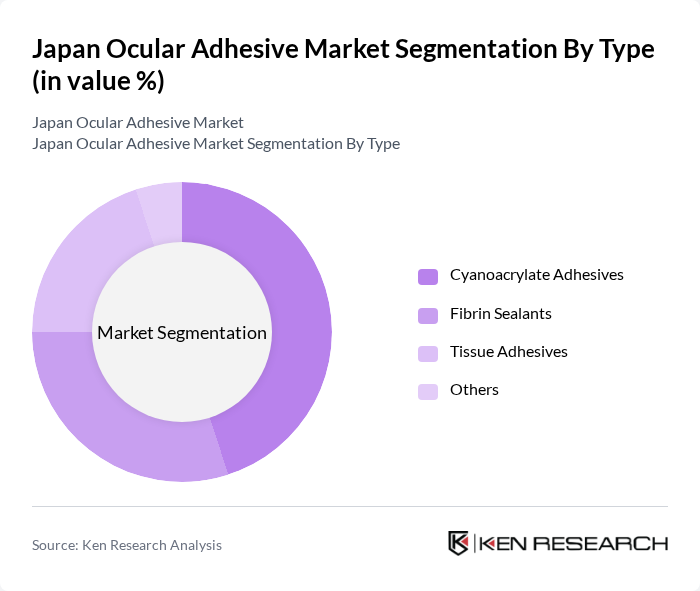
By Type:The market is segmented into various types of ocular adhesives, including Cyanoacrylate Adhesives, Fibrin Sealants, Tissue Adhesives, and Others. Cyanoacrylate adhesives are widely used due to their rapid bonding capabilities and ease of application, making them a preferred choice in surgical settings. Fibrin sealants are gaining traction for their biological compatibility and effectiveness in wound healing, while tissue adhesives are increasingly utilized in cosmetic procedures.
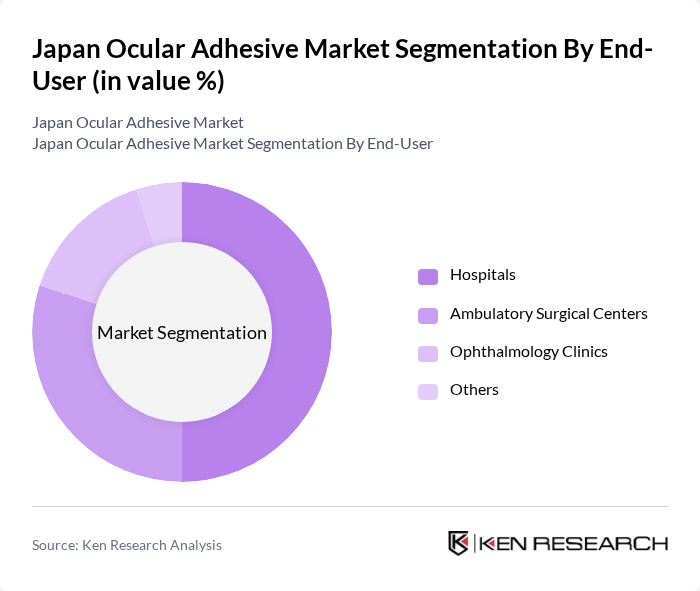
By End-User:The ocular adhesive market is categorized by end-users, including Hospitals, Ambulatory Surgical Centers, Ophthalmology Clinics, and Others. Hospitals are the largest end-users due to their comprehensive surgical services and advanced facilities. Ambulatory surgical centers are also significant as they provide specialized eye care services, while ophthalmology clinics cater to a growing number of patients seeking eye treatments.
The Japan Ocular Adhesive Market is characterized by a dynamic mix of regional and international players. Leading participants such as Johnson & Johnson Vision, Bausch + Lomb, Alcon, Medtronic, Santen Pharmaceutical, Ocular Therapeutix, Eyevance Pharmaceuticals, Aerie Pharmaceuticals, Novartis, AbbVie, Regeneron Pharmaceuticals, Genentech, Pfizer, Merck & Co., Takeda Pharmaceutical Company contribute to innovation, geographic expansion, and service delivery in this space.
The future of the ocular adhesive market in Japan appears promising, driven by ongoing technological advancements and a growing emphasis on patient-centric healthcare solutions. As healthcare infrastructure expands, particularly in rural areas, the accessibility of advanced ocular adhesives is expected to improve. Additionally, the increasing integration of digital technologies in healthcare will facilitate better patient management and education, further enhancing the adoption of innovative adhesive solutions in surgical practices.
| Segment | Sub-Segments |
|---|---|
| By Type | Cyanoacrylate Adhesives Fibrin Sealants Tissue Adhesives Others |
| By End-User | Hospitals Ambulatory Surgical Centers Ophthalmology Clinics Others |
| By Application | Surgical Procedures Trauma Care Cosmetic Procedures Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Others |
| By Region | Kanto Kansai Chubu Others |
| By Patient Demographics | Pediatric Patients Adult Patients Geriatric Patients Others |
| By Product Formulation | Liquid Adhesives Gel Adhesives Spray Adhesives Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Ophthalmic Surgeons | 100 | Ophthalmologists, Eye Surgeons |
| Hospital Procurement Managers | 80 | Procurement Officers, Supply Chain Managers |
| Patients Undergoing Eye Surgery | 75 | Post-operative Patients, Caregivers |
| Healthcare Policy Makers | 50 | Health Administrators, Policy Analysts |
| Medical Device Distributors | 60 | Sales Representatives, Distribution Managers |
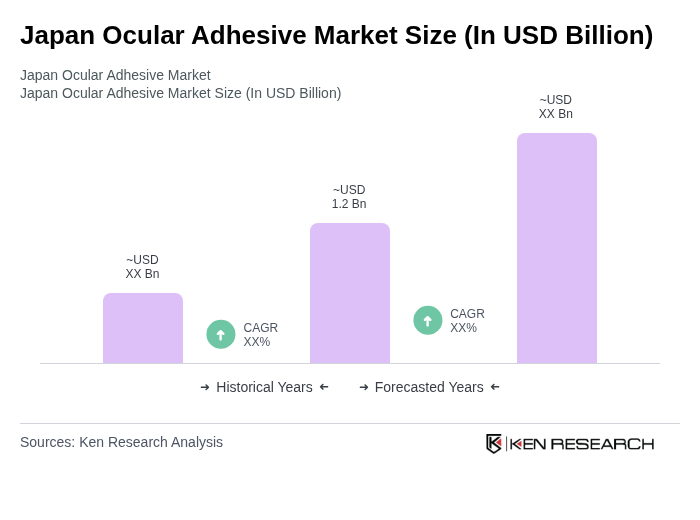
The Japan Ocular Adhesive Market is valued at approximately USD 1.2 billion, reflecting a significant growth trend driven by the increasing prevalence of ocular surgeries and advancements in adhesive technologies.