Region:Middle East
Author(s):Geetanshi
Product Code:KRAC8179
Pages:86
Published On:November 2025
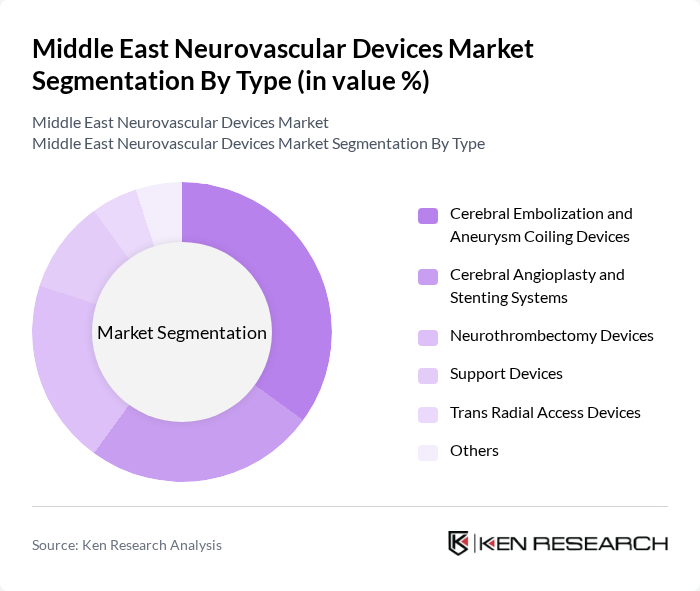
By Type:The market is segmented into various types of neurovascular devices, including cerebral embolization and aneurysm coiling devices, cerebral angioplasty and stenting systems, neurothrombectomy devices, support devices, trans radial access devices, and others. Among these, cerebral embolization and aneurysm coiling devices are leading the market due to their critical role in treating aneurysms and preventing strokes. The increasing incidence of cerebrovascular diseases and the growing preference for minimally invasive procedures are driving the demand for these devices. Cerebral embolization and aneurysm coiling devices held the largest segment share at 40.7 percent in 2024, followed by cerebral angioplasty and stenting systems and neurothrombectomy devices.
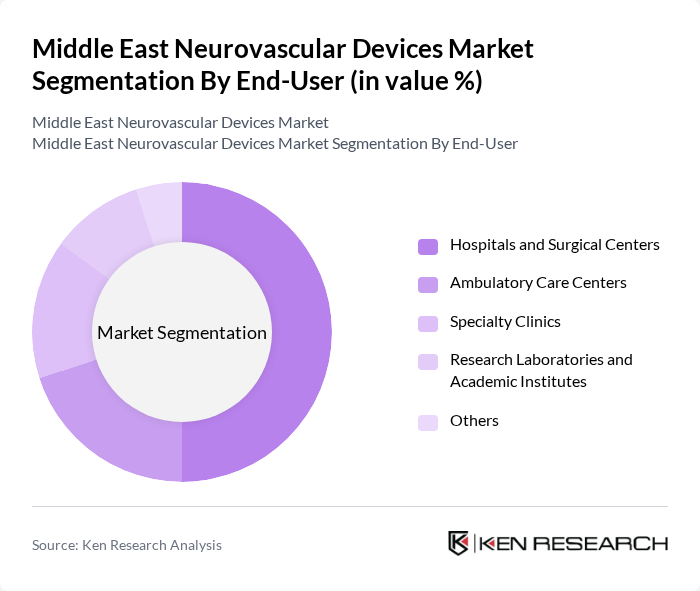
By End-User:The end-user segmentation includes hospitals and surgical centers, ambulatory care centers, specialty clinics, research laboratories and academic institutes, and others. Hospitals and surgical centers dominate this segment, accounting for a significant share due to their comprehensive facilities and advanced technologies for treating neurovascular conditions. The increasing number of surgical procedures and the growing focus on specialized care in hospitals are key factors contributing to this dominance. Hospitals and surgical centers held a 50 percent market share in 2024, reflecting their central role in neurovascular interventions.
The Middle East Neurovascular Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic, Stryker Corporation, Boston Scientific Corporation, Johnson & Johnson (Cerenovus), Terumo Corporation, Penumbra, Inc., Abbott Laboratories, MicroVention, Inc. (Terumo Group), Asahi Intecc Co., Ltd., Cook Medical, B. Braun Melsungen AG, Balt Extrusion, Phenox GmbH, Neuravi (acquired by J&J), Inari Medical contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Middle East neurovascular devices market appears promising, driven by ongoing advancements in technology and increasing healthcare investments. As governments prioritize healthcare infrastructure, the integration of innovative solutions, such as AI in diagnostics, is expected to enhance patient care. Additionally, the growing trend towards personalized medicine will likely lead to tailored treatment options, further propelling market growth and improving patient outcomes in the region.
| Segment | Sub-Segments |
|---|---|
| By Type | Cerebral Embolization and Aneurysm Coiling Devices Cerebral Angioplasty and Stenting Systems Neurothrombectomy Devices Support Devices Trans Radial Access Devices Others |
| By End-User | Hospitals and Surgical Centers Ambulatory Care Centers Specialty Clinics Research Laboratories and Academic Institutes Others |
| By Region | GCC Countries (Saudi Arabia, UAE, Qatar, Kuwait, Bahrain, Oman) Levant Region (Jordan, Lebanon, Iraq, Syria, Palestine) North Africa (Egypt, Morocco, Algeria, Tunisia) Rest of Middle East & Africa |
| By Application | Ischemic Stroke Treatment Aneurysm Treatment Arteriovenous Malformation & Fistula Treatment Intracerebral Hemorrhage Management Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Others |
| By Technology | Mechanical Devices Flow Diverters Bioactive Coils Hybrid Devices Others |
| By Policy Support | Government Subsidies Tax Incentives Research Grants Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Neurosurgery Device Utilization | 45 | Neurosurgeons, Interventional Radiologists |
| Hospital Procurement Practices | 50 | Procurement Managers, Supply Chain Directors |
| Clinical Trials and Research Insights | 40 | Clinical Researchers, Medical Device Innovators |
| Patient Outcomes and Satisfaction | 48 | Healthcare Administrators, Patient Care Coordinators |
| Market Trends and Innovations | 42 | Industry Analysts, Medical Device Consultants |
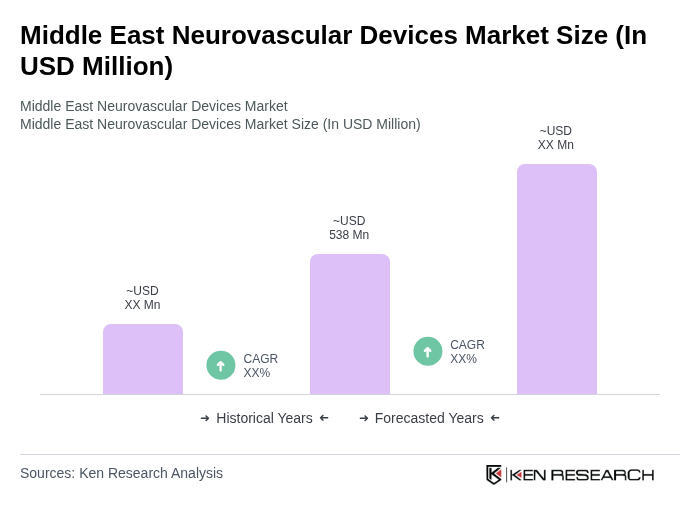
The Middle East Neurovascular Devices Market is valued at approximately USD 538 million, reflecting significant growth driven by the rising prevalence of neurovascular diseases and advancements in medical technology across the region.