Region:Middle East
Author(s):Dev
Product Code:KRAD5101
Pages:98
Published On:December 2025
By Type:
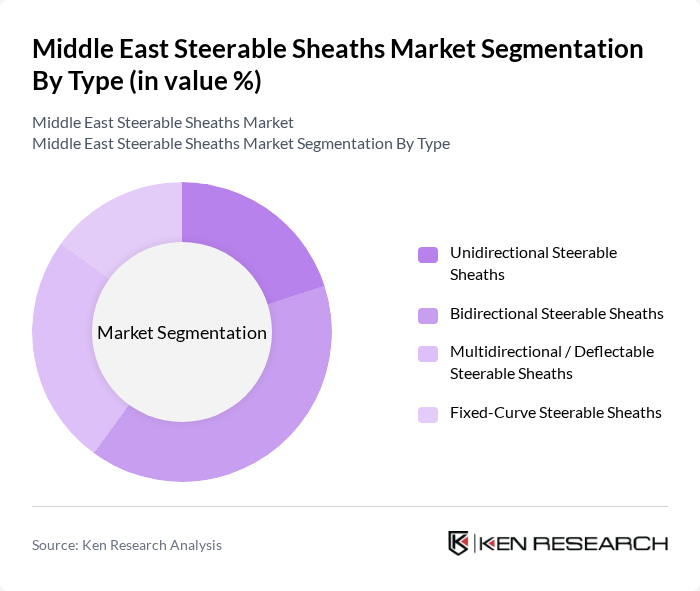
The market is segmented into four types: Unidirectional Steerable Sheaths, Bidirectional Steerable Sheaths, Multidirectional / Deflectable Steerable Sheaths, and Fixed-Curve Steerable Sheaths. This typology is consistent with global segmentation used in steerable sheath and steerable introducer sheath market reports, which differentiate products based on the directionality of deflection and control. Among these, Bidirectional Steerable Sheaths are dominating the market due to their versatility and ease of use in a wide range of electrophysiology and structural heart interventions, including atrial fibrillation ablation and left atrial appendage closure. They allow for precise navigation and stable positioning in challenging cardiac anatomies, which is crucial in complex procedures. The increasing adoption of these sheaths in cardiac and vascular interventions, supported by operator preference for improved torque control and reach, is driving their market share as healthcare providers seek to enhance procedural efficiency, reduce fluoroscopy time, and lower complication rates.
By End-User:
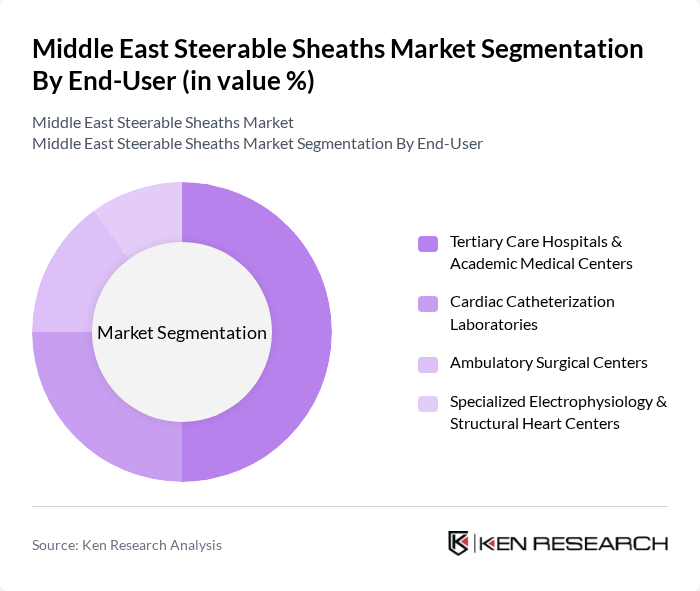
The end-user segmentation includes Tertiary Care Hospitals & Academic Medical Centers, Cardiac Catheterization Laboratories, Ambulatory Surgical Centers, and Specialized Electrophysiology & Structural Heart Centers. This structure reflects how global and regional steerable sheath market studies categorize demand by care setting and procedure complexity. Tertiary Care Hospitals & Academic Medical Centers are leading this segment due to their comprehensive cardiovascular and electrophysiology programs, higher patient volumes, and concentration of complex structural heart and arrhythmia procedures that routinely require advanced introducer and steerable sheath systems. These institutions are equipped with the latest imaging and navigation technologies, hybrid operating rooms, and highly trained interventional cardiologists and electrophysiologists, making them the preferred sites for complex interventions using steerable sheaths. The growing referral of patients with atrial fibrillation, heart failure, and valvular disease to these centers, along with increasing participation in clinical research and technology adoption programs, is further propelling their market dominance.
The Middle East Steerable Sheaths Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Boston Scientific Corporation, Abbott Laboratories (Abbott Vascular), Johnson & Johnson (Biosense Webster), Terumo Corporation, Cook Medical LLC, B. Braun Melsungen AG, Merit Medical Systems, Inc., Oscor Inc. (a Teleflex company), Baylis Medical (a Boston Scientific company), Biotronik SE & Co. KG, Teleflex Incorporated, CardioFocus, Inc., MicroPort Scientific Corporation, Osypka AG contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Middle East steerable sheaths market appears promising, driven by ongoing advancements in medical technology and a growing emphasis on patient-centric healthcare solutions. As healthcare infrastructure expands, particularly in emerging economies, the demand for innovative medical devices is expected to rise. Additionally, the integration of digital technologies into healthcare practices will likely enhance the efficiency and effectiveness of steerable sheath applications, paving the way for broader adoption and improved patient outcomes in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Type | Unidirectional Steerable Sheaths Bidirectional Steerable Sheaths Multidirectional / Deflectable Steerable Sheaths Fixed-Curve Steerable Sheaths |
| By End-User | Tertiary Care Hospitals & Academic Medical Centers Cardiac Catheterization Laboratories Ambulatory Surgical Centers Specialized Electrophysiology & Structural Heart Centers |
| By Application | Cardiac Electrophysiology Procedures (e.g., AF Ablation) Structural Heart Interventions (e.g., LAA Closure, TAVR Support) Peripheral Vascular Interventions Neurovascular and Other Interventional Procedures |
| By Material | Polyurethane-Based Steerable Sheaths Pebax and Other Thermoplastic Elastomers PTFE / Hydrophilic-Coated Sheaths Composite & Braided Reinforced Sheaths |
| By Region | GCC Countries (Saudi Arabia, UAE, Qatar, Kuwait, Oman, Bahrain) Levant (Jordan, Lebanon, Iraq, Others) Iran North Africa (Egypt and Rest of North Africa) Rest of Middle East |
| By Distribution Channel | Direct Sales to Hospitals and Cath Labs Regional Distributors / Importers Group Purchasing Organizations (GPOs) & Tender-Based Channels Online and E-Procurement Platforms |
| By Pricing Model | Per-Procedure Disposable Pricing Volume-Based / Tiered Discount Contracts Long-Term Tender & Framework Agreements Bundled Pricing with EP / Structural Heart Device Portfolios |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Cardiology Procedures | 100 | Cardiologists, Interventional Radiologists |
| Urology Applications | 80 | Urologists, Surgical Nurses |
| Gastroenterology Uses | 70 | Gastroenterologists, Endoscopy Technicians |
| Research & Development Insights | 60 | Medical Device Engineers, Product Managers |
| Healthcare Procurement | 90 | Procurement Managers, Supply Chain Analysts |
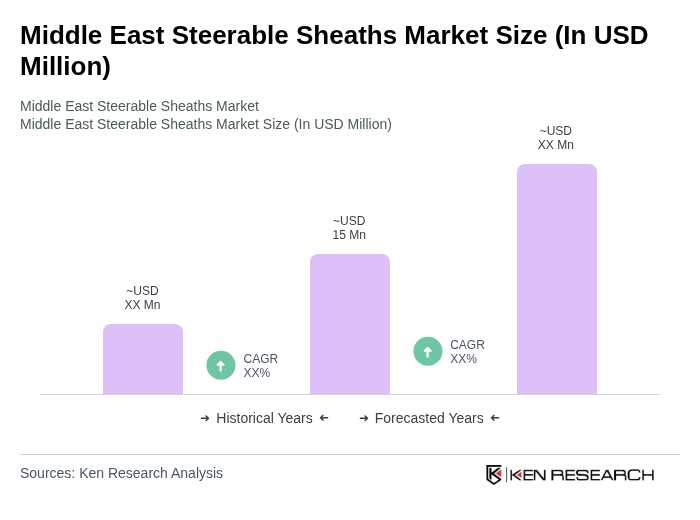
The Middle East Steerable Sheaths Market is valued at approximately USD 15 million, reflecting a historical analysis of the market and its share within the global steerable sheaths and introducer sheath markets.