Region:Middle East
Author(s):Shubham
Product Code:KRAD0810
Pages:85
Published On:August 2025
By Type:The market is segmented into various types of cardiovascular devices, including diagnostic and monitoring devices, therapeutic and surgical devices, imaging systems, and others. Each sub-segment plays a crucial role in addressing specific cardiovascular conditions and improving patient outcomes. Diagnostic and monitoring devices, such as ECGs, Holter monitors, blood pressure monitors, and remote cardiac monitoring systems, are increasingly adopted for early detection and continuous management of cardiovascular diseases. Therapeutic and surgical devices include stents, pacemakers, catheters, heart valves, implantable cardioverter defibrillators (ICDs), cardiac resynchronization therapy (CRT) devices, and electrophysiology devices, which are vital for advanced interventions and improved survival rates. Imaging systems, including echocardiography, MRI, and CT scanners, support accurate diagnosis and treatment planning.
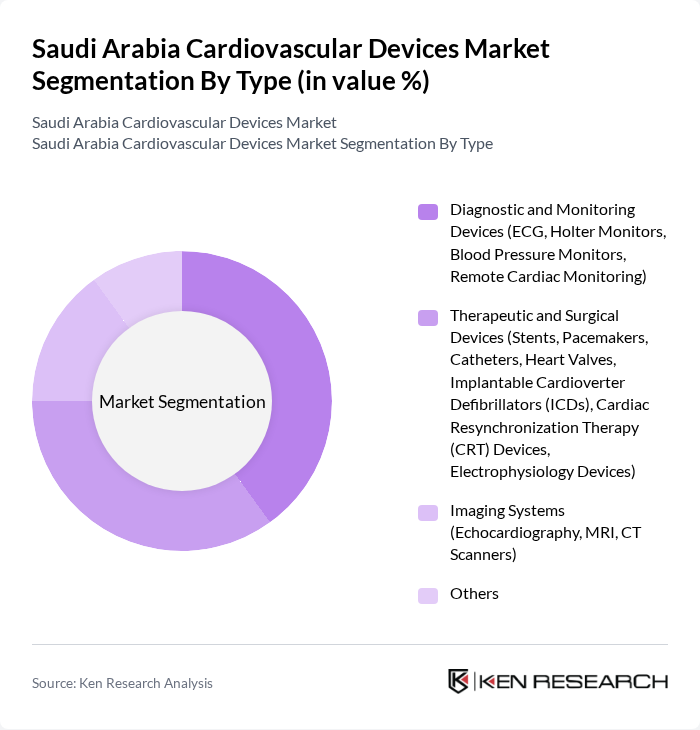
Thediagnostic and monitoring devicessegment is currently dominating the market due to the increasing emphasis on early detection and continuous monitoring of cardiovascular diseases. Devices such as ECGs and Holter monitors are widely used in hospitals and clinics, driven by the growing awareness of heart health among the population. The trend towards remote cardiac monitoring has also gained traction, particularly in the wake of the COVID-19 pandemic, as patients seek convenient and effective ways to manage their health from home.
By End-User:The market is segmented based on end-users, including hospitals, specialty centers & clinics, diagnostic centers, ambulatory surgical centers, and home healthcare. Each end-user category has unique requirements and contributes differently to the overall market dynamics. Hospitals are the primary end-users, leveraging advanced infrastructure and specialized cardiac units to deliver comprehensive cardiovascular care. Specialty centers and clinics focus on targeted interventions and outpatient services, while diagnostic centers and ambulatory surgical centers provide essential support for early detection and minimally invasive procedures. Home healthcare is a growing segment, reflecting the shift towards remote monitoring and patient-centric care.
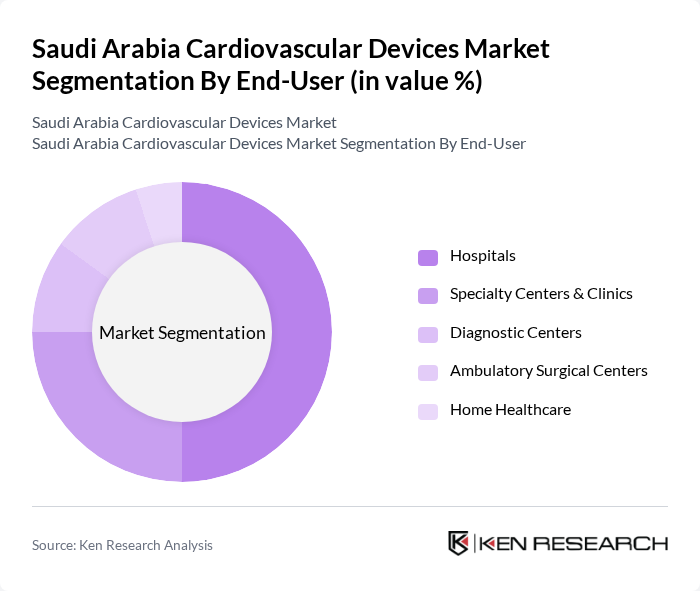
Hospitalsare the leading end-user segment, accounting for a significant share of the market. This dominance is attributed to the high volume of cardiovascular procedures performed in these facilities, coupled with the availability of advanced medical technologies. Hospitals invest heavily in state-of-the-art cardiovascular devices to enhance patient care and improve surgical outcomes. The increasing number of specialized cardiac units within hospitals further supports this trend, as they require a wide range of devices for diagnosis and treatment.
The Saudi Arabia Cardiovascular Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Abbott Laboratories, Boston Scientific Corporation, Johnson & Johnson MedTech, Siemens Healthineers AG, Philips Healthcare (Royal Philips), Edwards Lifesciences Corporation, Biotronik SE & Co. KG, Terumo Corporation, Stryker Corporation, B. Braun Melsungen AG, Cook Medical LLC, MicroPort Scientific Corporation, GE HealthCare, LivaNova PLC contribute to innovation, geographic expansion, and service delivery in this space.
The future of the cardiovascular devices market in Saudi Arabia appears promising, driven by ongoing technological advancements and government support. The integration of artificial intelligence in diagnostics and treatment planning is expected to enhance patient outcomes significantly. Furthermore, the shift towards preventive healthcare will likely lead to increased demand for cardiovascular monitoring devices, fostering innovation and collaboration among local and international firms to meet evolving healthcare needs.
| Segment | Sub-Segments |
|---|---|
| By Type | Diagnostic and Monitoring Devices (ECG, Holter Monitors, Blood Pressure Monitors, Remote Cardiac Monitoring) Therapeutic and Surgical Devices (Stents, Pacemakers, Catheters, Heart Valves, Implantable Cardioverter Defibrillators (ICDs), Cardiac Resynchronization Therapy (CRT) Devices, Electrophysiology Devices) Imaging Systems (Echocardiography, MRI, CT Scanners) Others |
| By End-User | Hospitals Specialty Centers & Clinics Diagnostic Centers Ambulatory Surgical Centers Home Healthcare |
| By Distribution Channel | Direct Sales Distributors Online Sales |
| By Application | Coronary Artery Disease Heart Rhythm Disorders (Arrhythmias, Atrial Fibrillation, Bradycardia) Heart Failure Hypertension Others |
| By Region | Central Region (Riyadh) Eastern Region Western Region Southern Region |
| By Price Range | Low-End Devices Mid-Range Devices High-End Devices |
| By Technology | Conventional Devices Advanced Digital Devices (Wireless, Remote Monitoring, AI-enabled) Wearable Devices |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Cardiovascular Device Procurement | 100 | Hospital Procurement Managers, Medical Device Buyers |
| Cardiologists and Heart Specialists | 80 | Cardiologists, Interventional Cardiologists |
| Medical Device Distributors | 60 | Sales Managers, Distribution Executives |
| Healthcare Policy Makers | 40 | Health Ministry Officials, Regulatory Affairs Managers |
| Patient Advocacy Groups | 40 | Patient Representatives, Health Advocates |
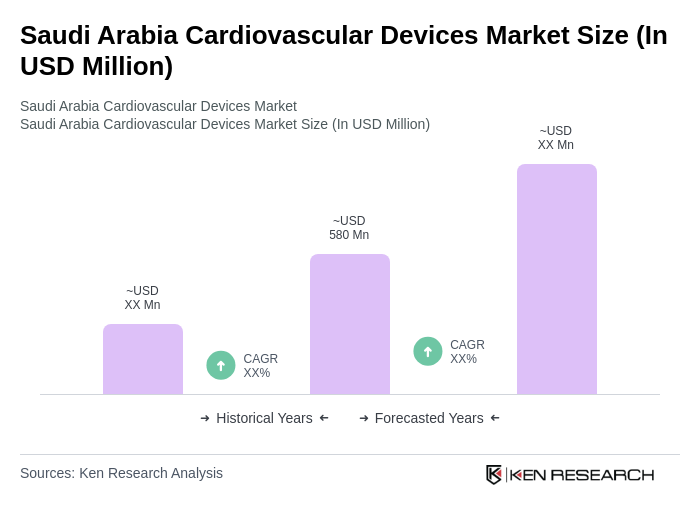
The Saudi Arabia Cardiovascular Devices Market is valued at approximately USD 580 million, reflecting a significant growth driven by the rising prevalence of cardiovascular diseases and advancements in medical technology.