Region:Middle East
Author(s):Geetanshi
Product Code:KRAD3821
Pages:89
Published On:November 2025
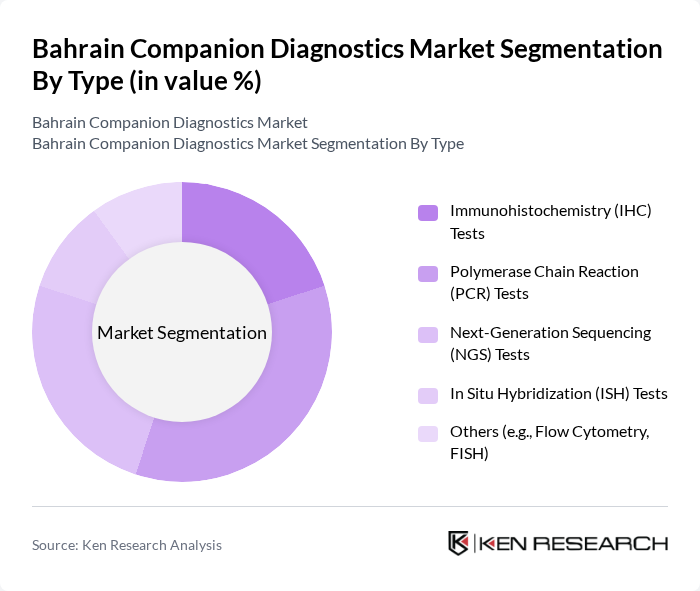
By Type:The market is segmented into various types of diagnostic tests, including Immunohistochemistry (IHC) Tests, Polymerase Chain Reaction (PCR) Tests, Next-Generation Sequencing (NGS) Tests, In Situ Hybridization (ISH) Tests, and Others (e.g., Flow Cytometry, FISH). Among these, PCR Tests are currently dominating the market due to their high sensitivity and specificity, making them essential for detecting genetic mutations and infectious diseases. The increasing adoption of PCR technology in clinical laboratories and hospitals is driven by the need for rapid and accurate diagnostic results, particularly in oncology and infectious disease management.
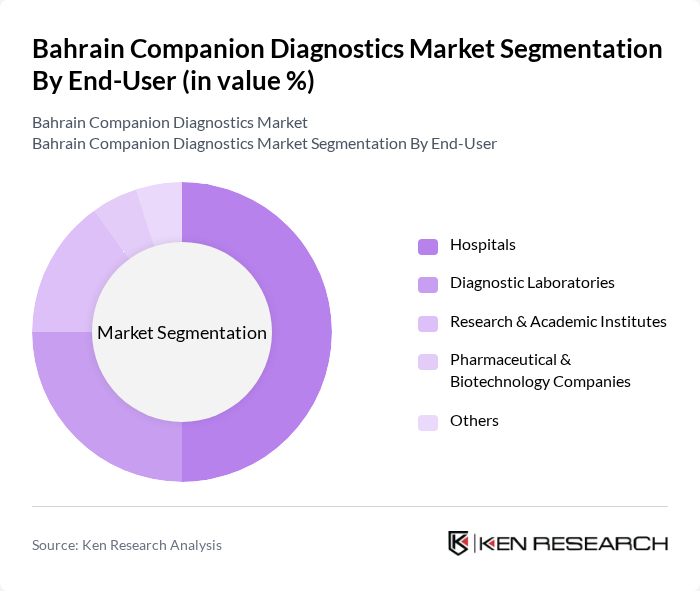
By End-User:The end-user segmentation includes Hospitals, Diagnostic Laboratories, Research & Academic Institutes, Pharmaceutical & Biotechnology Companies, and Others. Hospitals are the leading end-users in the market, primarily due to their extensive patient base and the critical role they play in disease diagnosis and management. The increasing investment in hospital infrastructure and the growing emphasis on personalized medicine are driving the demand for companion diagnostics in these settings, enabling healthcare providers to offer tailored treatment options.
The Bahrain Companion Diagnostics Market is characterized by a dynamic mix of regional and international players. Leading participants such as Roche Diagnostics, Abbott Laboratories, Agilent Technologies, Thermo Fisher Scientific, QIAGEN, Illumina, Siemens Healthineers, Bio-Rad Laboratories, Hologic, Myriad Genetics, Genomic Health, Becton, Dickinson and Company, PerkinElmer, Luminex Corporation, Exact Sciences Corporation contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Bahrain companion diagnostics market appears promising, driven by ongoing advancements in technology and a growing emphasis on precision medicine. As healthcare infrastructure expands, the integration of artificial intelligence in diagnostics is expected to enhance accuracy and efficiency. Furthermore, increased collaboration between pharmaceutical companies and diagnostic developers will likely lead to innovative solutions, ultimately improving patient outcomes and fostering a more robust healthcare ecosystem in Bahrain.
| Segment | Sub-Segments |
|---|---|
| By Type | Immunohistochemistry (IHC) Tests Polymerase Chain Reaction (PCR) Tests Next-Generation Sequencing (NGS) Tests In Situ Hybridization (ISH) Tests Others (e.g., Flow Cytometry, FISH) |
| By End-User | Hospitals Diagnostic Laboratories Research & Academic Institutes Pharmaceutical & Biotechnology Companies Others |
| By Disease Type | Oncology (Cancer) Infectious Diseases Neurological Disorders Cardiovascular Diseases Others (e.g., Autoimmune Disorders) |
| By Technology | PCR-based Tests Next-Generation Sequencing Immunohistochemistry In Situ Hybridization Others (e.g., Mass Spectrometry, Microarray) |
| By Distribution Channel | Direct Sales Distributor Sales Online Sales Others |
| By Region | Northern Governorate Southern Governorate Capital Governorate Muharraq Governorate Others |
| By Policy Support | Government Subsidies Tax Incentives Research Grants Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Oncology Companion Diagnostics | 60 | Oncologists, Clinical Researchers |
| Cardiovascular Diagnostics | 50 | Cardiologists, Laboratory Technicians |
| Genetic Testing Services | 40 | Genetic Counselors, Healthcare Administrators |
| Infectious Disease Diagnostics | 40 | Infectious Disease Specialists, Microbiologists |
| Regulatory and Compliance Insights | 40 | Regulatory Affairs Managers, Quality Assurance Officers |
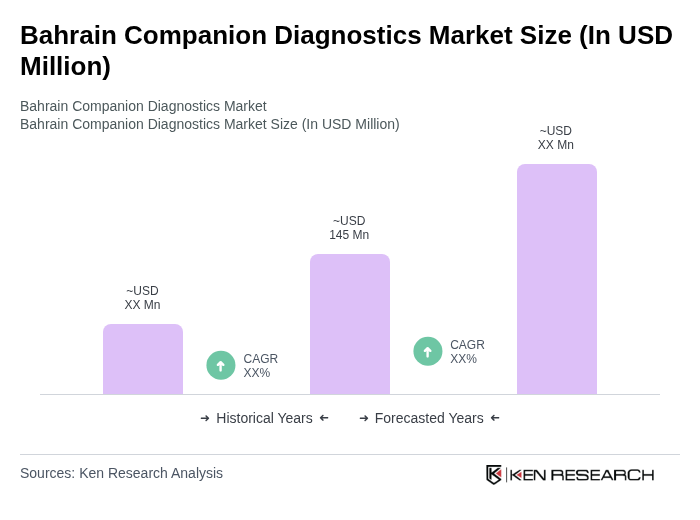
The Bahrain Companion Diagnostics Market is valued at approximately USD 145 million, reflecting a significant growth driven by the increasing prevalence of chronic diseases and advancements in personalized medicine.