Region:Europe
Author(s):Geetanshi
Product Code:KRAB5703
Pages:90
Published On:October 2025
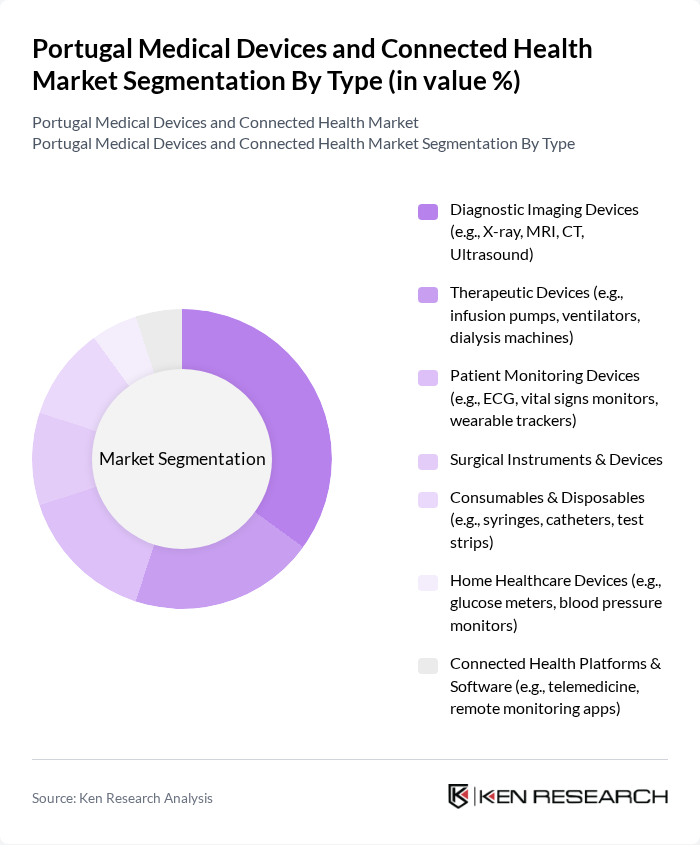
By Type:The market is segmented into various types of medical devices and connected health solutions. The subsegments include Diagnostic Imaging Devices, Therapeutic Devices, Patient Monitoring Devices, Surgical Instruments & Devices, Consumables & Disposables, Home Healthcare Devices, and Connected Health Platforms & Software. Among these, Diagnostic Imaging Devices are currently leading the market due to their essential role in early disease detection and management, driven by technological advancements and increasing demand for non-invasive diagnostic methods. The importance of early diagnoses is a key factor sustaining demand for imaging equipment such as X-ray, MRI, and CT devices. The market is also seeing rising adoption of patient monitoring devices, including wearables, and connected health platforms that integrate with electronic health records and telemedicine services.
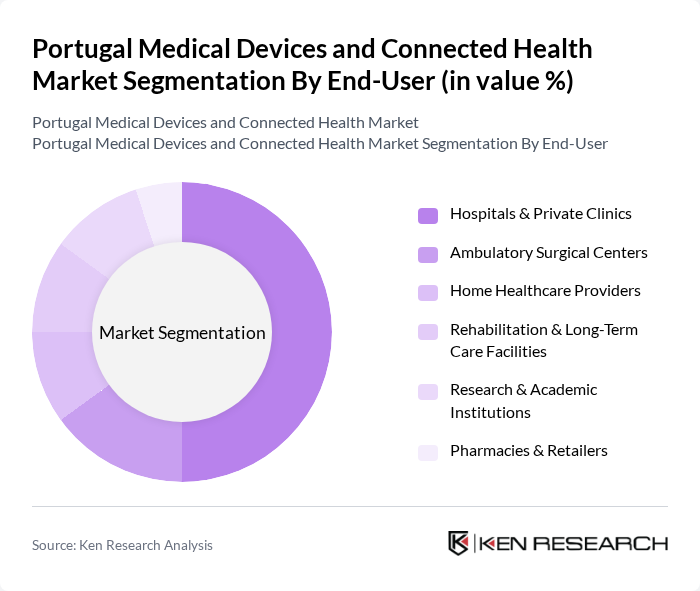
By End-User:The market is segmented by end-users, including Hospitals & Private Clinics, Ambulatory Surgical Centers, Home Healthcare Providers, Rehabilitation & Long-Term Care Facilities, Research & Academic Institutions, and Pharmacies & Retailers. Hospitals & Private Clinics dominate the market due to their high demand for advanced medical devices and connected health solutions to improve patient care and operational efficiency. The shift toward outpatient and home-based care is gradually increasing the share of home healthcare providers and connected health platforms, though hospitals remain the primary end-user segment.
The Portugal Medical Devices and Connected Health Market is characterized by a dynamic mix of regional and international players. Leading participants such as B. Braun Medical S.A., Siemens Healthineers Portugal, Philips Healthcare Portugal, Medtronic Portugal, GE Healthcare Portugal, Johnson & Johnson Medical Devices Portugal, Abbott Laboratories Portugal, Stryker Portugal, Boston Scientific Portugal, Zimmer Biomet Portugal, Terumo Europe N.V. (Portugal), Olympus Medical Systems Portugal, Canon Medical Systems Portugal, Roche Diagnostics Portugal, Fresenius Kabi Portugal, Hologic Portugal, Mindray Medical Portugal, Glintt Healthcare Solutions, Lusíadas Saúde, José de Mello Saúde contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Portugal medical devices and connected health market appears promising, driven by ongoing technological innovations and a growing emphasis on preventive healthcare. As the healthcare landscape evolves, the integration of AI and telehealth services will likely enhance patient engagement and streamline care delivery. Additionally, the increasing focus on personalized medicine will further shape the market, encouraging the development of tailored medical solutions that address individual patient needs and preferences, ultimately improving health outcomes.
| Segment | Sub-Segments |
|---|---|
| By Type | Diagnostic Imaging Devices (e.g., X-ray, MRI, CT, Ultrasound) Therapeutic Devices (e.g., infusion pumps, ventilators, dialysis machines) Patient Monitoring Devices (e.g., ECG, vital signs monitors, wearable trackers) Surgical Instruments & Devices Consumables & Disposables (e.g., syringes, catheters, test strips) Home Healthcare Devices (e.g., glucose meters, blood pressure monitors) Connected Health Platforms & Software (e.g., telemedicine, remote monitoring apps) |
| By End-User | Hospitals & Private Clinics Ambulatory Surgical Centers Home Healthcare Providers Rehabilitation & Long-Term Care Facilities Research & Academic Institutions Pharmacies & Retailers |
| By Distribution Channel | Direct Sales (Manufacturers to End-User) Local Distributors/Importers Online Marketplaces & E-commerce Hospital Group Purchasing Organizations (GPOs) Retail Pharmacies |
| By Application | Cardiovascular Care Orthopedics & Trauma Neurology & Mental Health Diabetes & Chronic Disease Management Oncology Respiratory Care Others |
| By Component | Hardware (Physical Devices) Software (Health IT, Apps, Analytics) Services (Maintenance, Integration, Support) |
| By Price Range | Low-End Devices Mid-Range Devices High-End Devices |
| By Policy Support | Subsidies for Medical Device Manufacturers Tax Incentives for R&D Grants for Digital Health Initiatives |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Healthcare Providers | 60 | Doctors, Nurses, Hospital Administrators |
| Medical Device Manufacturers | 40 | Product Managers, R&D Directors |
| Connected Health Solution Providers | 40 | Technology Officers, Business Development Managers |
| Patients and Caregivers | 50 | Patients using connected health devices, Caregivers |
| Regulatory Bodies | 40 | Regulatory Affairs Specialists, Compliance Officers |
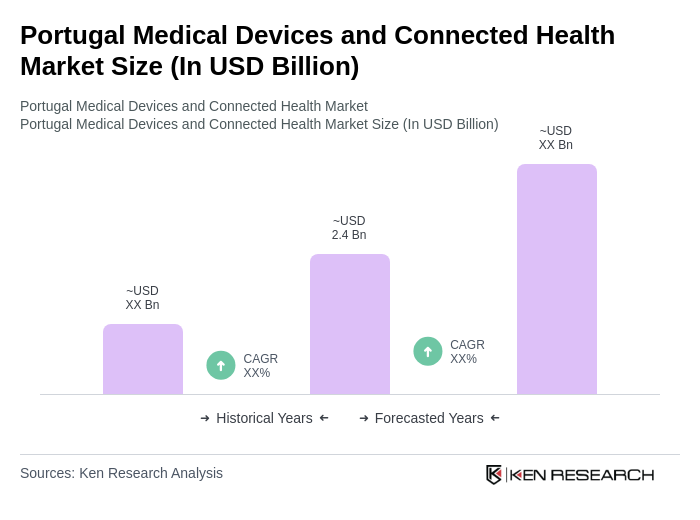
The Portugal Medical Devices and Connected Health Market is valued at approximately USD 2.4 billion, driven by factors such as an aging population, increasing chronic diseases, and advancements in technology that enhance patient care and monitoring.