Region:Asia
Author(s):Geetanshi
Product Code:KRAE4986
Pages:92
Published On:December 2025
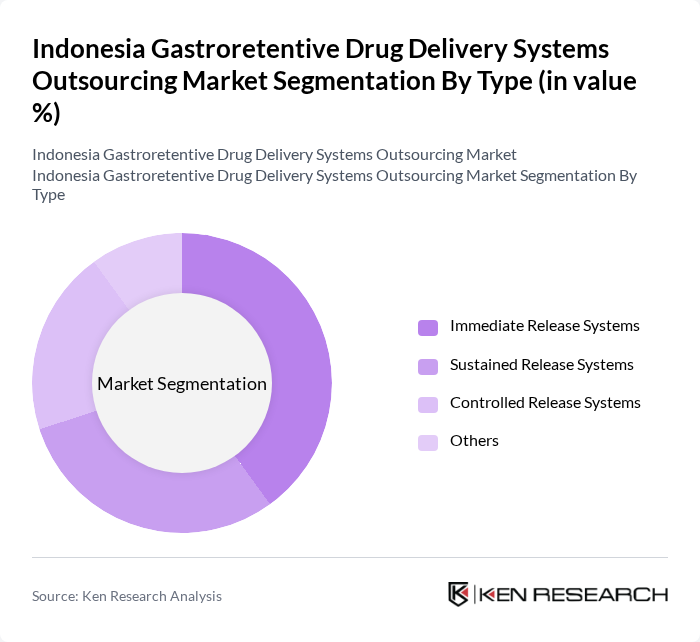
By Type:The market is segmented into various types of gastroretentive drug delivery systems, including Immediate Release Systems, Sustained Release Systems, Controlled Release Systems, and Others. Among these, Immediate Release Systems are gaining traction due to their rapid onset of action, which is crucial for managing acute conditions. Sustained Release Systems are also popular as they provide prolonged therapeutic effects, enhancing patient compliance.
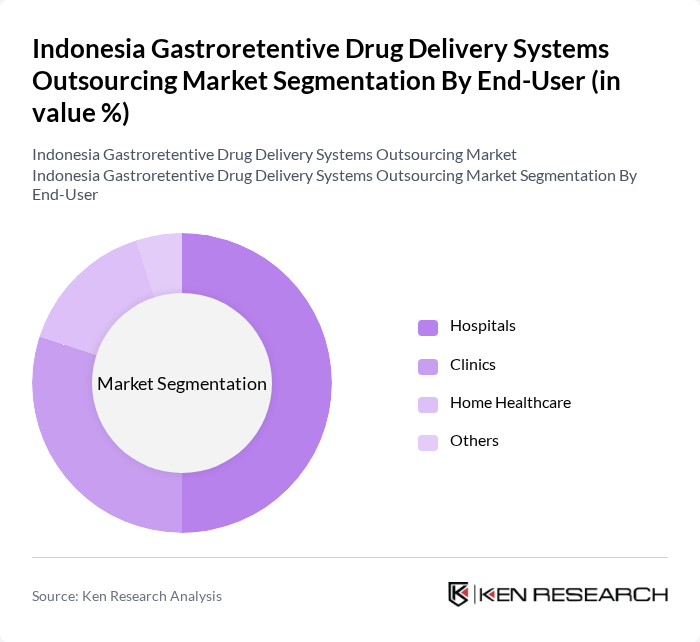
By End-User:The end-user segmentation includes Hospitals, Clinics, Home Healthcare, and Others. Hospitals are the leading end-users due to their extensive patient base and the need for advanced drug delivery systems to manage complex medical conditions. Clinics are also significant as they cater to outpatient services, while Home Healthcare is emerging as a growing segment due to the increasing trend of at-home patient care.
The Indonesia Gastroretentive Drug Delivery Systems Outsourcing Market is characterized by a dynamic mix of regional and international players. Leading participants such as PT Kalbe Farma Tbk, PT Kimia Farma Tbk, PT Indofarma Tbk, PT Merck Tbk, PT Sanofi Indonesia, PT Pfizer Indonesia, PT Novartis Indonesia, PT AstraZeneca Indonesia, PT GlaxoSmithKline Indonesia, PT Bayer Indonesia, PT Sandoz Indonesia, PT Abbott Indonesia, PT Johnson & Johnson Indonesia, PT Roche Indonesia, PT Takeda Indonesia contribute to innovation, geographic expansion, and service delivery in this space.
The future of the gastroretentive drug delivery systems market in Indonesia appears promising, driven by technological advancements and increasing healthcare investments. As the government continues to prioritize healthcare improvements, the integration of digital health technologies is expected to enhance patient engagement and treatment outcomes. Furthermore, the growing focus on personalized medicine will likely lead to tailored drug delivery solutions, addressing the unique needs of patients and fostering market growth in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Type | Immediate Release Systems Sustained Release Systems Controlled Release Systems Others |
| By End-User | Hospitals Clinics Home Healthcare Others |
| By Application | Pain Management Cardiovascular Diseases Diabetes Management Others |
| By Distribution Channel | Online Pharmacies Retail Pharmacies Hospital Pharmacies Others |
| By Region | Java Sumatra Bali Others |
| By Drug Formulation | Tablets Capsules Liquids Others |
| By Manufacturing Process | Extrusion-Spheronization Hot Melt Extrusion Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Pharmaceutical Manufacturers | 100 | Product Development Managers, Regulatory Affairs Specialists |
| Healthcare Providers | 80 | Pharmacists, Physicians, Clinical Researchers |
| Market Analysts | 60 | Healthcare Market Analysts, Industry Consultants |
| Patients with Chronic Conditions | 75 | Chronic Disease Patients, Caregivers |
| Regulatory Bodies | 50 | Health Policy Makers, Regulatory Officers |
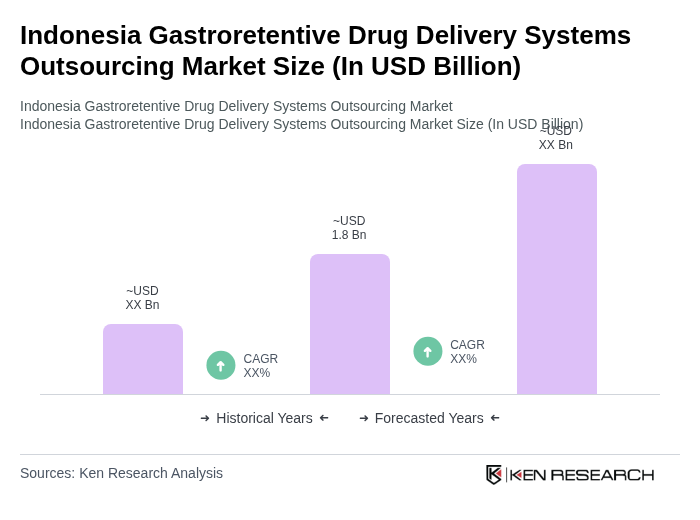
The Indonesia Gastroretentive Drug Delivery Systems Outsourcing Market is valued at approximately USD 1.8 billion, reflecting significant growth driven by the rising prevalence of chronic diseases and the demand for advanced drug delivery systems.