Region:Middle East
Author(s):Rebecca
Product Code:KRAE3469
Pages:80
Published On:February 2026
By Type:The market can be segmented into various types of hemostatic instruments, including Surgical Hemostatic Agents, Mechanical Hemostatic Devices, Biological Hemostatic Products, and Others. Each of these subsegments plays a crucial role in surgical procedures, with specific applications and benefits.
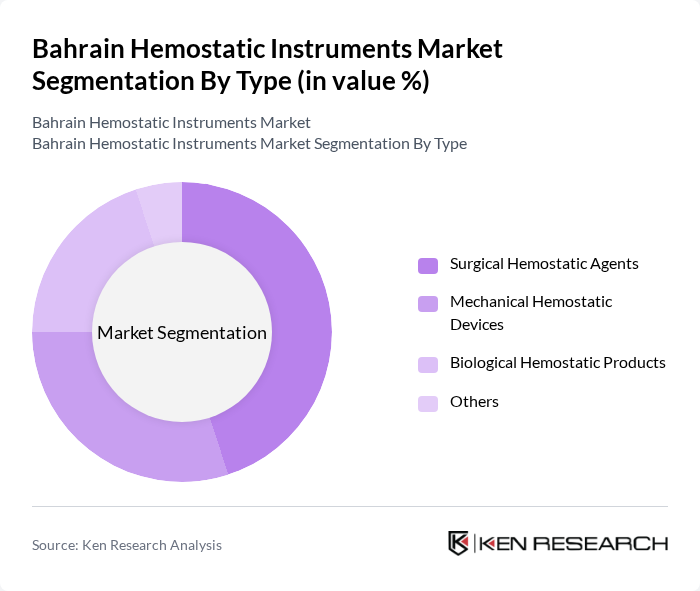
The Surgical Hemostatic Agents subsegment is currently dominating the market due to their widespread use in various surgical procedures. These agents are preferred for their effectiveness in controlling bleeding and are often used in emergency situations. The increasing number of surgeries, coupled with advancements in surgical techniques, has led to a higher demand for these agents. Additionally, the growing awareness among healthcare professionals regarding the importance of effective hemostasis is further driving the adoption of surgical hemostatic agents.
By End-User:The market can also be segmented based on end-users, which include Hospitals, Ambulatory Surgical Centers, Specialty Clinics, and Others. Each end-user category has distinct requirements and preferences for hemostatic instruments.
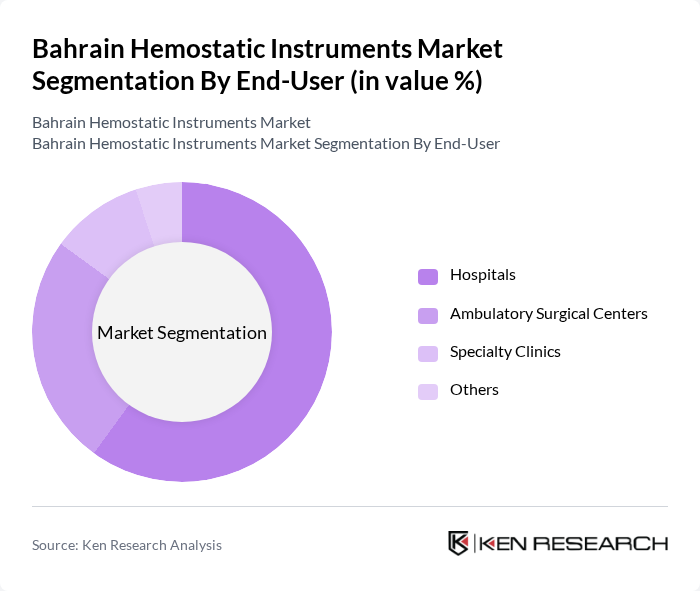
Hospitals are the leading end-user segment in the market, accounting for a significant share due to their extensive surgical operations and the need for a wide range of hemostatic instruments. The increasing number of surgical procedures performed in hospitals, along with the growing emphasis on patient safety and effective bleeding control, has resulted in a higher demand for hemostatic products. Furthermore, hospitals often have the resources to invest in advanced hemostatic technologies, further solidifying their position as the dominant end-user in the market.
The Bahrain Hemostatic Instruments Market is characterized by a dynamic mix of regional and international players. Leading participants such as Johnson & Johnson, Medtronic, B. Braun Melsungen AG, Ethicon (a subsidiary of Johnson & Johnson), Stryker Corporation, Baxter International Inc., C.R. Bard, Inc., Terumo Corporation, Hemostasis, LLC, Z-Medica, LLC, Cohera Medical, Inc., Integra LifeSciences, Acelity L.P. Inc., Medline Industries, Inc., Vascular Solutions, Inc. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Bahrain hemostatic instruments market appears promising, driven by ongoing advancements in medical technology and an increasing focus on patient safety. As healthcare providers continue to adopt innovative solutions, the integration of artificial intelligence in surgical procedures is expected to enhance precision and outcomes. Additionally, the expansion of healthcare infrastructure will facilitate greater access to advanced hemostatic instruments, ultimately improving surgical care and patient recovery rates in the region.
| Segment | Sub-Segments |
|---|---|
| By Type | Surgical Hemostatic Agents Mechanical Hemostatic Devices Biological Hemostatic Products Others |
| By End-User | Hospitals Ambulatory Surgical Centers Specialty Clinics Others |
| By Application | Cardiovascular Surgery Orthopedic Surgery General Surgery Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Others |
| By Region | Northern Governorate Southern Governorate Capital Governorate Others |
| By Product Formulation | Powder Formulations Liquid Formulations Gel Formulations Others |
| By Regulatory Approval Status | CE Marked Products FDA Approved Products Products Under Review Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Surgical Hemostatic Instruments | 100 | Surgeons, Operating Room Managers |
| Topical Hemostatic Agents | 80 | Pharmacists, Medical Supply Buyers |
| Emergency Medical Services Equipment | 60 | EMS Directors, Paramedics |
| Hospital Procurement Strategies | 90 | Procurement Officers, Supply Chain Managers |
| Clinical Trials and Research | 70 | Clinical Researchers, Medical Device Innovators |
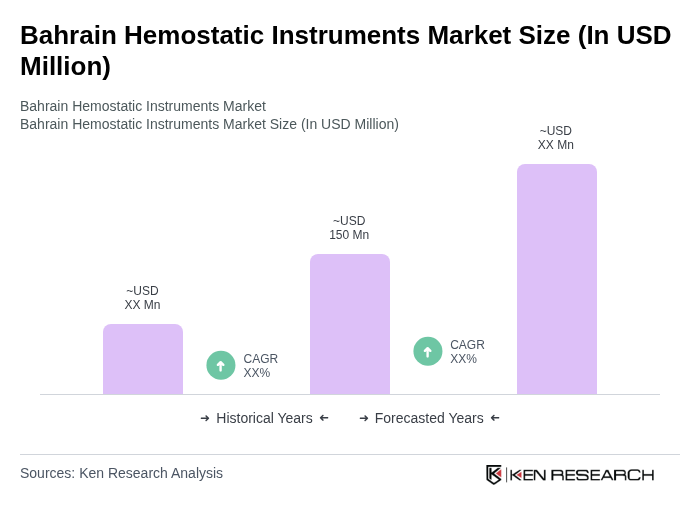
The Bahrain Hemostatic Instruments Market is valued at approximately USD 150 million, reflecting a five-year historical analysis. This valuation is influenced by the increasing prevalence of surgical procedures and advancements in medical technology.