Region:Asia
Author(s):Rebecca
Product Code:KRAE3472
Pages:81
Published On:February 2026
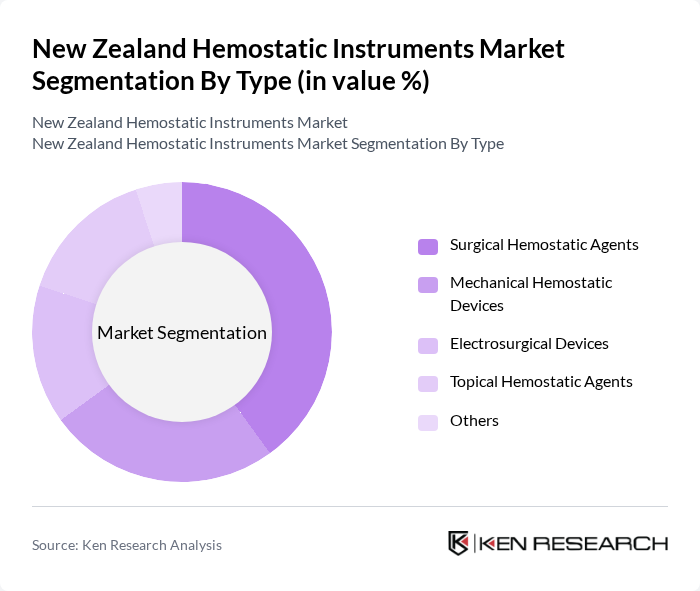
By Type:The hemostatic instruments market can be segmented into various types, including Surgical Hemostatic Agents, Mechanical Hemostatic Devices, Electrosurgical Devices, Topical Hemostatic Agents, and Others. Among these, Surgical Hemostatic Agents are gaining significant traction due to their effectiveness in controlling bleeding during surgical procedures. The increasing number of surgeries performed annually, coupled with advancements in surgical techniques, has led to a higher demand for these agents. Mechanical Hemostatic Devices are also witnessing growth as they provide reliable solutions for blood management in various surgical settings.
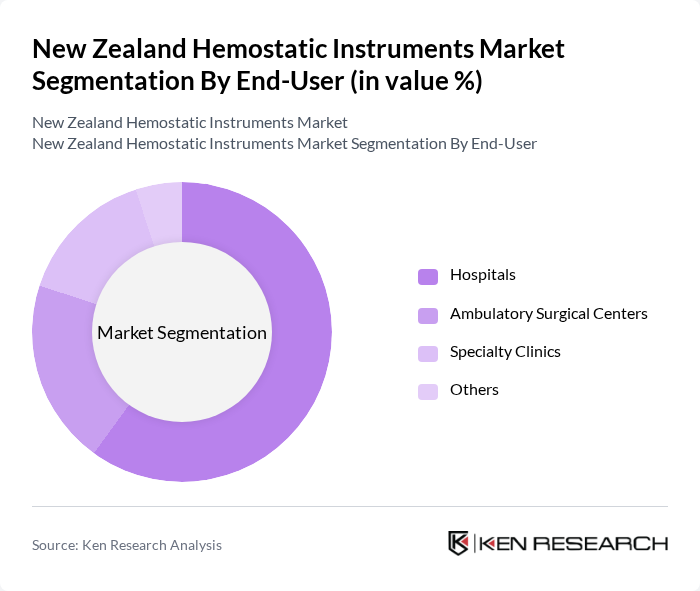
By End-User:The market can also be segmented based on end-users, which include Hospitals, Ambulatory Surgical Centers, Specialty Clinics, and Others. Hospitals are the primary end-users of hemostatic instruments, driven by the high volume of surgical procedures performed in these facilities. The increasing number of surgical interventions, along with the need for effective blood management, has led to a growing demand for hemostatic instruments in hospitals. Ambulatory Surgical Centers are also emerging as significant users due to the rise in outpatient surgeries.
The New Zealand Hemostatic Instruments Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic, Johnson & Johnson, B. Braun Melsungen AG, Ethicon, Stryker Corporation, Baxter International Inc., Terumo Corporation, C.R. Bard, Inc., Hemostasis, Inc., Z-Medica, LLC, Cohera Medical, Inc., Integra LifeSciences, Acelity, Vascular Solutions, Inc., Medline Industries, Inc. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the New Zealand hemostatic instruments market appears promising, driven by ongoing technological advancements and an increasing focus on patient-centered care. As healthcare providers prioritize minimally invasive procedures, the demand for innovative hemostatic solutions is expected to rise. Additionally, the integration of artificial intelligence in surgical practices is anticipated to enhance operational efficiency and patient outcomes, further propelling market growth in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Type | Surgical Hemostatic Agents Mechanical Hemostatic Devices Electrosurgical Devices Topical Hemostatic Agents Others |
| By End-User | Hospitals Ambulatory Surgical Centers Specialty Clinics Others |
| By Application | Cardiovascular Surgery Orthopedic Surgery General Surgery Trauma Surgery Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Others |
| By Region | North Island South Island Others |
| By Product Formulation | Liquid Formulations Powder Formulations Gel Formulations Others |
| By Technology | Conventional Technologies Advanced Technologies Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Hospital Procurement Departments | 100 | Procurement Managers, Supply Chain Coordinators |
| Emergency Medical Services | 75 | Paramedics, EMS Directors |
| Surgical Units in Hospitals | 90 | Surgeons, Surgical Nurses |
| Medical Device Distributors | 60 | Sales Representatives, Distribution Managers |
| Clinical Research Organizations | 50 | Clinical Researchers, Regulatory Affairs Specialists |
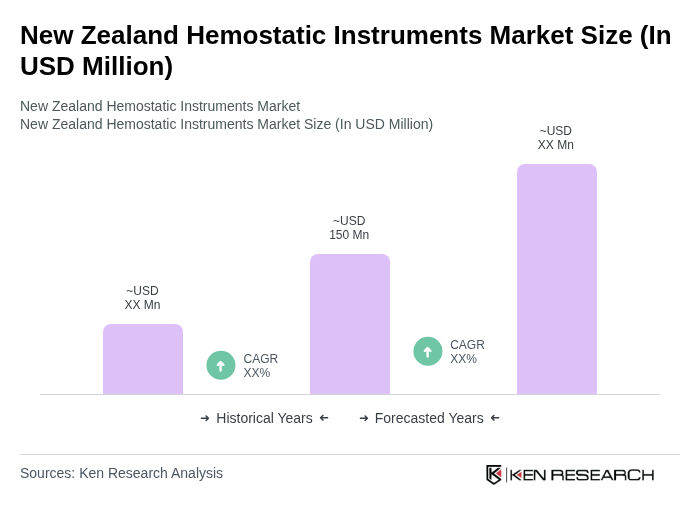
The New Zealand Hemostatic Instruments Market is valued at approximately USD 150 million, reflecting a five-year historical analysis that highlights growth driven by increased surgical procedures and advancements in medical technology.