Region:Middle East
Author(s):Rebecca
Product Code:KRAE3468
Pages:99
Published On:February 2026
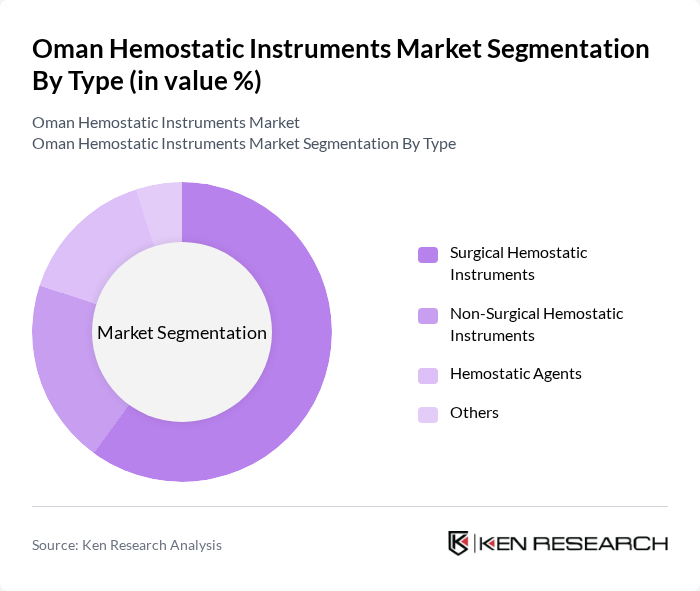
By Type:The market is segmented into Surgical Hemostatic Instruments, Non-Surgical Hemostatic Instruments, Hemostatic Agents, and Others. Surgical Hemostatic Instruments dominate the market due to their essential role in various surgical procedures, ensuring effective blood control and minimizing complications. The increasing number of surgeries performed annually, coupled with advancements in surgical techniques, drives the demand for these instruments. Non-Surgical Hemostatic Instruments and Hemostatic Agents are also gaining traction, particularly in emergency care settings.
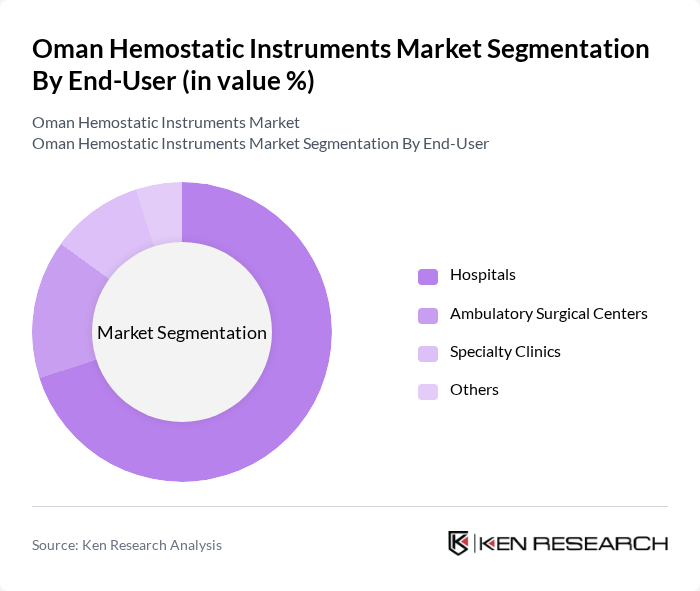
By End-User:The market is categorized into Hospitals, Ambulatory Surgical Centers, Specialty Clinics, and Others. Hospitals are the leading end-users, accounting for a significant portion of the market share. This dominance is attributed to the high volume of surgical procedures conducted in hospitals, which necessitates the use of advanced hemostatic instruments. Ambulatory Surgical Centers are also witnessing growth due to the increasing trend of outpatient surgeries, while Specialty Clinics cater to specific surgical needs.
The Oman Hemostatic Instruments Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic, Johnson & Johnson, B. Braun Melsungen AG, Stryker Corporation, Boston Scientific, Ethicon (a subsidiary of Johnson & Johnson), Terumo Corporation, Smith & Nephew, Zimmer Biomet, ConMed Corporation, Cook Medical, 3M Health Care, Halyard Health, Integra LifeSciences, Aesculap (a B. Braun company) contribute to innovation, geographic expansion, and service delivery in this space.
The Oman Hemostatic Instruments Market is poised for significant growth, driven by increasing surgical procedures and advancements in medical technology. The integration of artificial intelligence in surgical practices is expected to enhance precision and patient safety. Additionally, the focus on personalized medicine will likely lead to tailored hemostatic solutions, improving surgical outcomes. As healthcare infrastructure expands, the market will benefit from increased accessibility and innovation, positioning Oman as a key player in the regional medical device landscape.
| Segment | Sub-Segments |
|---|---|
| By Type | Surgical Hemostatic Instruments Non-Surgical Hemostatic Instruments Hemostatic Agents Others |
| By End-User | Hospitals Ambulatory Surgical Centers Specialty Clinics Others |
| By Application | Cardiovascular Surgery Orthopedic Surgery General Surgery Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Others |
| By Material Type | Metal Plastic Composite Materials Others |
| By Region | Muscat Dhofar Al Batinah Others |
| By Policy Support | Government Subsidies Tax Incentives Research Grants Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Hospital Procurement Departments | 100 | Procurement Managers, Supply Chain Coordinators |
| Surgeons and Medical Practitioners | 80 | Orthopedic Surgeons, General Surgeons |
| Medical Device Distributors | 60 | Sales Representatives, Distribution Managers |
| Healthcare Policy Makers | 50 | Health Administrators, Policy Analysts |
| Clinical Research Organizations | 40 | Clinical Researchers, Data Analysts |
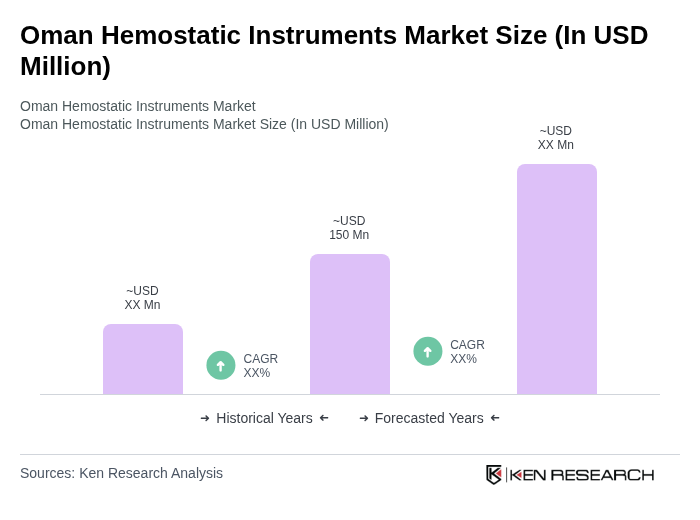
The Oman Hemostatic Instruments Market is valued at approximately USD 150 million, reflecting a significant growth trend driven by the increasing number of surgical procedures and advancements in medical technology.