Region:Central and South America
Author(s):Shubham
Product Code:KRAB5045
Pages:83
Published On:October 2025
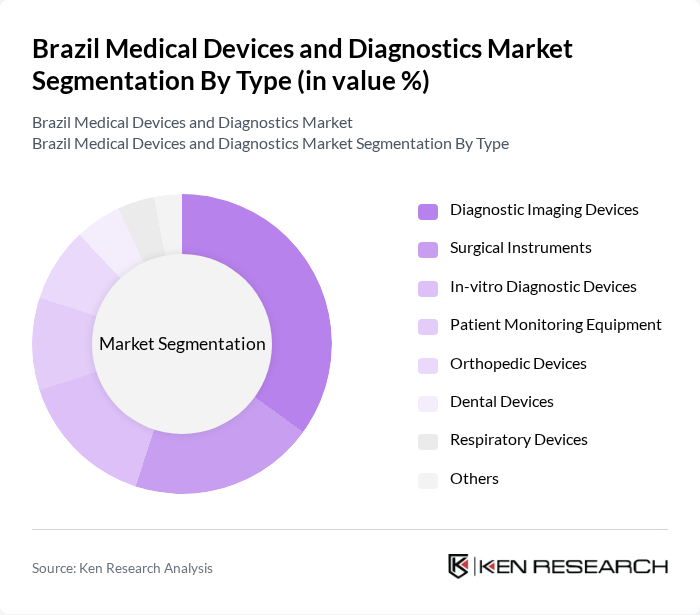
By Type:The market is segmented into various types of medical devices and diagnostics, including diagnostic imaging devices, surgical instruments, in-vitro diagnostic devices, patient monitoring equipment, orthopedic devices, dental devices, respiratory devices, and others. Among these, diagnostic imaging devices are leading the market due to their critical role in early disease detection and management. The increasing prevalence of chronic diseases, digital transformation of healthcare, and the demand for non-invasive diagnostic methods are driving the growth of this segment. Imports account for a significant share of imaging equipment, and mobile imaging solutions are increasingly important for regional access .
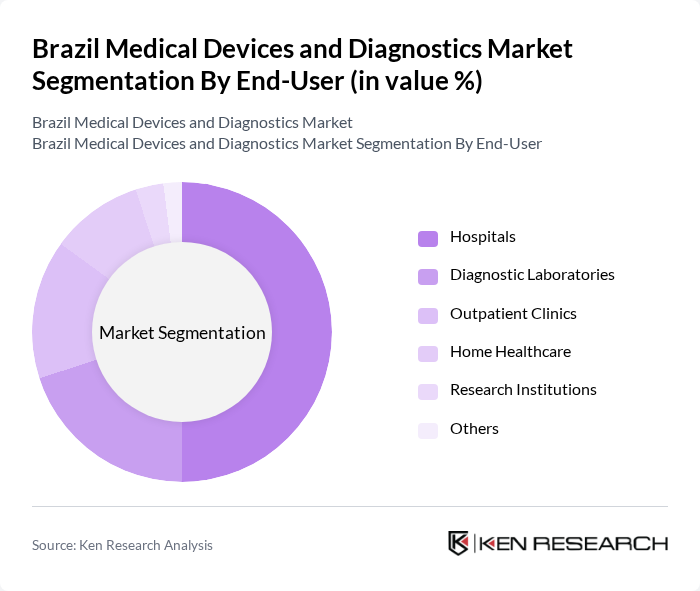
By End-User:The end-user segmentation includes hospitals, diagnostic laboratories, outpatient clinics, home healthcare, research institutions, and others. Hospitals are the dominant end-user segment, driven by the increasing number of healthcare facilities, rising demand for advanced medical technologies, and significant investments in public and private healthcare infrastructure. The focus on improving patient care and operational efficiency in hospitals is propelling the adoption of various medical devices and diagnostics. Home healthcare is also a rapidly growing segment due to the shift towards decentralized care and telemedicine .
The Brazil Medical Devices and Diagnostics Market is characterized by a dynamic mix of regional and international players. Leading participants such as Siemens Healthineers, GE Healthcare, Philips Healthcare, Medtronic, Abbott Laboratories, Johnson & Johnson, Becton, Dickinson and Company, Boston Scientific, Stryker Corporation, Roche Diagnostics, Thermo Fisher Scientific, Cardinal Health, 3M Health Care, Zimmer Biomet, Hologic, Inc., Mindray Medical do Brasil, Fanem Ltda., WEM Equipamentos Eletrônicos, Lifemed Industrial de Equipamentos e Artigos Médicos e Hospitalares S/A, Baumer S.A. contribute to innovation, geographic expansion, and service delivery in this space.
The future of Brazil's medical devices and diagnostics market appears promising, driven by ongoing healthcare reforms and technological innovations. The government’s commitment to improving healthcare infrastructure is expected to enhance access to medical services, particularly in underserved areas. Additionally, the integration of digital health solutions and AI technologies will likely transform diagnostics and patient care, fostering a more efficient healthcare ecosystem. As these trends continue, the market is poised for significant growth, attracting investments and enhancing patient outcomes.
| Segment | Sub-Segments |
|---|---|
| By Type | Diagnostic Imaging Devices Surgical Instruments In-vitro Diagnostic Devices Patient Monitoring Equipment Orthopedic Devices Dental Devices Respiratory Devices Others |
| By End-User | Hospitals Diagnostic Laboratories Outpatient Clinics Home Healthcare Research Institutions Others |
| By Application | Cardiovascular Applications Neurology Applications Orthopedic Applications Respiratory Applications Diabetes Management Cancer Diagnostics Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Retail Pharmacies Others |
| By Price Range | Low-End Devices Mid-Range Devices High-End Devices Others |
| By Technology | Digital Technology Analog Technology Hybrid Technology Others |
| By Regulatory Compliance | ANVISA Approved Devices CE Marked Devices FDA Approved Devices Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Diagnostic Imaging Devices | 100 | Radiologists, Imaging Technicians |
| Surgical Instruments | 60 | Surgeons, Operating Room Managers |
| In-vitro Diagnostics | 70 | Laboratory Managers, Pathologists |
| Wearable Health Devices | 50 | Healthcare Innovators, Product Managers |
| Patient Monitoring Systems | 65 | Nurses, Clinical Engineers |
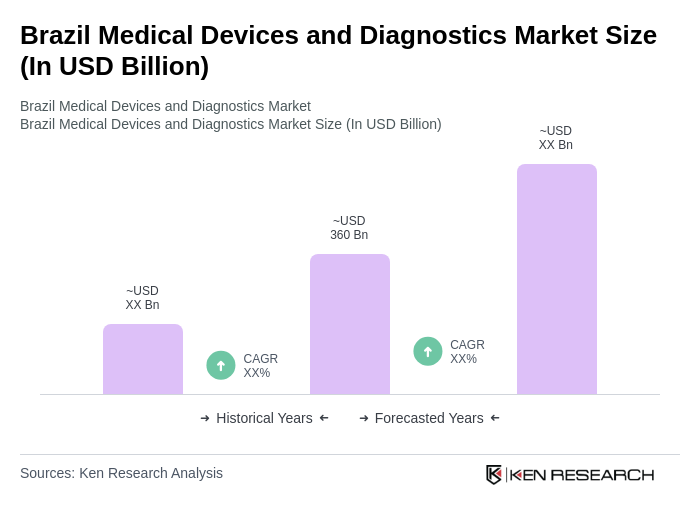
The Brazil Medical Devices and Diagnostics Market is valued at approximately USD 360 billion, reflecting significant growth driven by factors such as an aging population, increased healthcare expenditure, and advancements in medical technology.