Region:Middle East
Author(s):Geetanshi
Product Code:KRAD8230
Pages:93
Published On:December 2025
 Market.png)
By Technique:The market is segmented into various techniques, including Immunochemistry, Molecular Diagnostics, Hematology, Microbiology, Histochemistry, and Self-Blood Glucose Testing. Among these, Immunochemistry is currently the leading technique due to its widespread application in disease diagnosis and monitoring, particularly in hormone and tumor marker testing. The increasing adoption of automated immunoassay systems in laboratories is further driving its dominance.

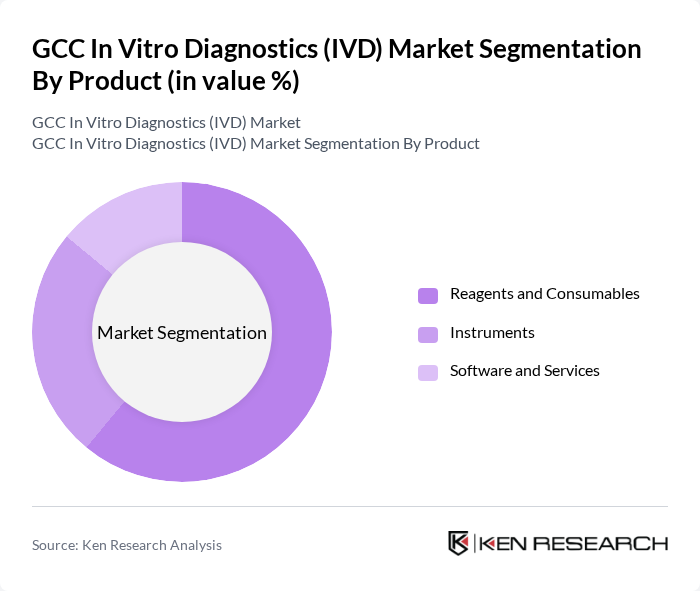
By Product:The product segmentation includes Reagents and Consumables, Instruments, and Software and Services. Reagents and Consumables dominate the market due to their essential role in diagnostic testing processes. The increasing demand for high-quality reagents, coupled with the rise in laboratory testing, has solidified their position as the leading product category in the IVD market.
The GCC In Vitro Diagnostics (IVD) Market is characterized by a dynamic mix of regional and international players. Leading participants such as Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, Thermo Fisher Scientific, Becton, Dickinson and Company, Bio-Rad Laboratories, QIAGEN, Hologic, Inc., Ortho Clinical Diagnostics, Agilent Technologies, PerkinElmer, Inc., Sysmex Corporation, Mindray Medical International Limited, DiaSorin S.p.A., Grifols S.A., Guardant Health, Wellesta (VECanDx) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the GCC IVD market appears promising, driven by ongoing advancements in technology and increasing healthcare investments. In future, the integration of telemedicine and remote diagnostics is expected to enhance access to IVD solutions, particularly in underserved areas. Additionally, the growing focus on personalized medicine will likely lead to the development of tailored diagnostic tests, improving patient outcomes and fostering innovation within the industry.
| Segment | Sub-Segments |
|---|---|
| By Technique | Immunochemistry Molecular Diagnostics Hematology Microbiology Histochemistry Self-Blood Glucose Testing |
| By Product | Reagents and Consumables Instruments Software and Services |
| By Usability | Disposable IVD Devices Reusable IVD Devices |
| By Application | Infectious Diseases Diabetes Cancer/Oncology Cardiology Autoimmune Disease Other Applications |
| By End-User | Diagnostic Laboratories Hospitals and Clinics Home-Care and Self-Testing Settings Other End Users |
| By Diagnostic Approach | Point-of-Care Diagnostics Centralized Laboratory-Based Diagnostics |
| By Region | Saudi Arabia UAE Qatar Kuwait Oman Bahrain |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Clinical Laboratories | 120 | Laboratory Managers, Clinical Pathologists |
| Hospitals and Healthcare Providers | 100 | Healthcare Administrators, Procurement Officers |
| IVD Manufacturers | 80 | Product Managers, R&D Directors |
| Regulatory Bodies | 50 | Regulatory Affairs Specialists, Compliance Officers |
| Distributors and Wholesalers | 70 | Sales Managers, Supply Chain Coordinators |
The GCC In Vitro Diagnostics (IVD) Market is valued at approximately USD 2.1 billion, driven by the rising prevalence of chronic diseases, advancements in diagnostic technologies, and an increasing focus on preventive healthcare in the region.