Region:Europe
Author(s):Geetanshi
Product Code:KRAA3225
Pages:83
Published On:September 2025
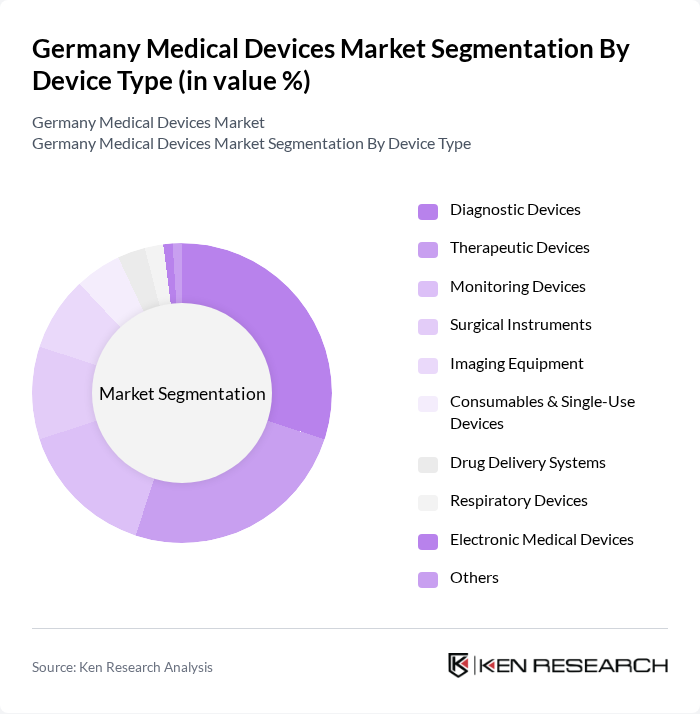
By Device Type:The medical devices market can be segmented into various device types, including diagnostic devices, therapeutic devices, monitoring devices, surgical instruments, imaging equipment, consumables & single-use devices, drug delivery systems, respiratory devices, electronic medical devices, and others. Among these, diagnostic devices are currently leading the market due to the rising demand for early disease detection and preventive healthcare measures. The increasing prevalence of chronic diseases and the need for regular health monitoring have further propelled the growth of this segment. Additionally, single-use and disposable devices are experiencing accelerated growth, driven by infection control requirements and efficiency in clinical settings .
By End-User:The end-user segmentation includes hospitals, clinics, ambulatory surgery centers, homecare settings, rehabilitation centers, long-term care centers, specialty clinics, and research institutions. Hospitals are the dominant end-user segment, driven by the increasing number of surgical procedures and the need for advanced medical technologies to enhance patient care. The growing trend of outpatient services and the expansion of healthcare facilities also contribute to the rising demand for medical devices in hospitals. Homecare settings are also experiencing robust growth, supported by the shift toward decentralized healthcare and remote patient monitoring .

The Germany Medical Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Siemens Healthineers AG, B. Braun Melsungen AG, Fresenius Medical Care AG & Co. KGaA, Drägerwerk AG & Co. KGaA, Carl Zeiss Meditec AG, Philips Medizin Systeme GmbH, Medtronic GmbH, Johnson & Johnson Medical GmbH, Stryker GmbH, GE Healthcare Deutschland, Abbott GmbH & Co. KG, Olympus Europa SE & Co. KG, Terumo Deutschland GmbH, Smith & Nephew GmbH, Boston Scientific Medizintechnik GmbH contribute to innovation, geographic expansion, and service delivery in this space.
The future of the German medical devices market appears promising, driven by ongoing technological advancements and a growing emphasis on personalized healthcare solutions. As the healthcare landscape evolves, the integration of AI and machine learning into medical devices will enhance diagnostic accuracy and treatment efficacy. Additionally, the shift towards value-based care will encourage the development of cost-effective medical technologies, ensuring that patient outcomes remain at the forefront of healthcare delivery in Germany.
| Segment | Sub-Segments |
|---|---|
| By Device Type | Diagnostic Devices Therapeutic Devices Monitoring Devices Surgical Instruments Imaging Equipment Consumables & Single-Use Devices Drug Delivery Systems Respiratory Devices Electronic Medical Devices Others |
| By End-User | Hospitals Clinics Ambulatory Surgery Centers Homecare Settings Rehabilitation Centers Long Term Care Centers Specialty Clinics Research Institutions |
| By Application | Cardiovascular Orthopedics Neurology Oncology Dental Ophthalmology General Surgery Respiratory ENT (Ear, Nose, and Throat) Nephrology & Urology Others |
| By Distribution Channel | Direct Sales Third Party Distributors Online Sales |
| By Region | North Germany South Germany East Germany West Germany |
| By Price Range | Low-End Devices Mid-Range Devices High-End Devices |
| By Technology | Digital Health Technologies Robotics D Printing Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Diagnostic Imaging Devices | 60 | Radiologists, Imaging Technologists |
| Surgical Instruments | 50 | Surgeons, Operating Room Managers |
| Orthopedic Devices | 40 | Orthopedic Surgeons, Physical Therapists |
| Cardiovascular Devices | 50 | Cardiologists, Cardiac Nurses |
| Wearable Health Technology | 40 | Health Tech Innovators, Product Managers |
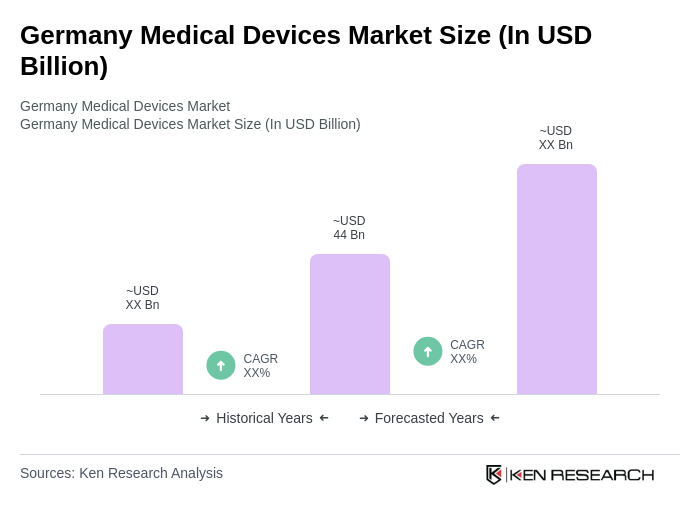
The Germany Medical Devices Market is valued at approximately USD 44 billion, driven by technological advancements, an aging population, and increased healthcare expenditure. This market is expected to continue growing as demand for innovative medical devices rises.