Region:Asia
Author(s):Geetanshi
Product Code:KRAB5837
Pages:93
Published On:October 2025
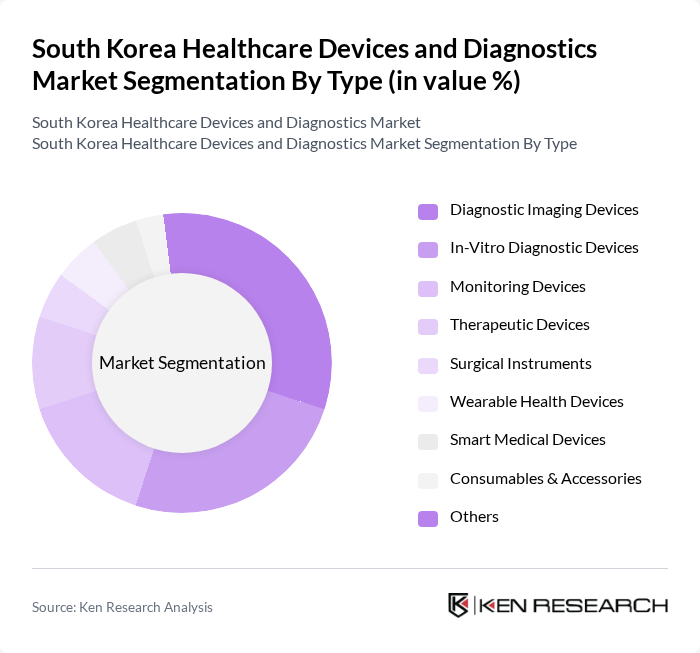
By Type:The market is segmented into various types of healthcare devices and diagnostics, including diagnostic imaging devices, in-vitro diagnostic devices, monitoring devices, therapeutic devices, surgical instruments, wearable health devices, smart medical devices, consumables & accessories, and others. Among these, diagnostic imaging devices and in-vitro diagnostic devices are particularly prominent due to their critical role in disease detection and management. The increasing prevalence of chronic diseases, the adoption of molecular and AI-driven diagnostics, and the demand for early diagnosis are driving the growth of these segments. Smart medical devices and wearable health devices are also experiencing rapid uptake, supported by digital health trends and patient self-monitoring initiatives .
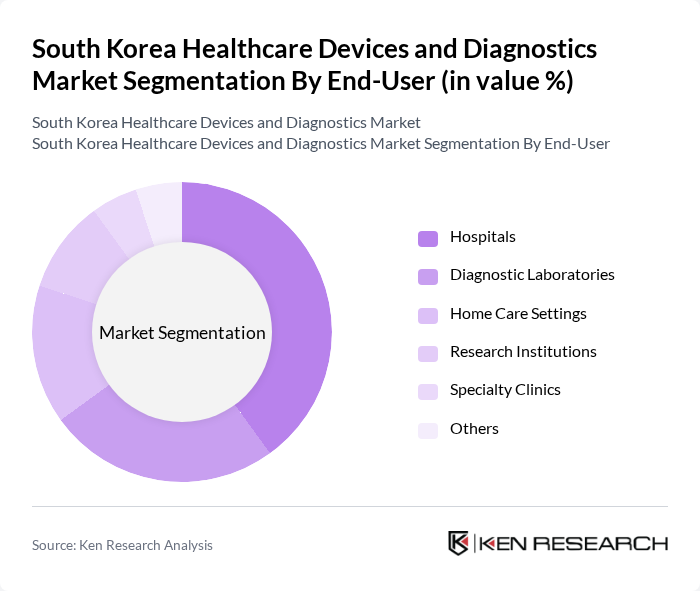
By End-User:The healthcare devices and diagnostics market is further segmented by end-users, including hospitals, diagnostic laboratories, home care settings, research institutions, specialty clinics, and others. Hospitals are the leading end-user segment, driven by the increasing number of patients requiring advanced diagnostic and therapeutic services. Diagnostic laboratories are expanding their role, particularly in molecular and in-vitro diagnostics, while the growing trend of home healthcare and self-monitoring is gaining traction as patients prefer receiving care in the comfort of their homes .
The South Korea Healthcare Devices and Diagnostics Market is characterized by a dynamic mix of regional and international players. Leading participants such as Samsung Medison Co., Ltd., LG Electronics Inc., Medtronic Korea Ltd., Siemens Healthineers Korea, GE Healthcare Korea, Philips Korea Ltd., Roche Diagnostics Korea Co., Ltd., Abbott Korea Ltd., Johnson & Johnson Medical Korea Ltd., B. Braun Korea Ltd., Stryker Korea Ltd., Olympus Korea Co., Ltd., Terumo Korea Co., Ltd., Hologic Korea Ltd., Canon Medical Systems Korea Co., Ltd., SD Biosensor, Inc., Seegene Inc., Osang Healthcare Co., Ltd., Humasis Co., Ltd., Green Cross Medical Science Corp. (GC MS) contribute to innovation, geographic expansion, and service delivery in this space.
The South Korea healthcare devices and diagnostics market is poised for significant transformation, driven by ongoing technological innovations and a growing emphasis on personalized medicine. As digital health solutions gain traction, the integration of artificial intelligence in diagnostics is expected to enhance accuracy and efficiency. Furthermore, the expansion of healthcare services into rural areas will likely improve access to essential medical devices, fostering a more inclusive healthcare environment. These trends indicate a dynamic future for the market, with opportunities for growth and development.
| Segment | Sub-Segments |
|---|---|
| By Type | Diagnostic Imaging Devices In-Vitro Diagnostic Devices Monitoring Devices Therapeutic Devices Surgical Instruments Wearable Health Devices Smart Medical Devices Consumables & Accessories Others |
| By End-User | Hospitals Diagnostic Laboratories Home Care Settings Research Institutions Specialty Clinics Others |
| By Application | Cardiovascular Applications Neurological Applications Orthopedic Applications Respiratory Applications Oncology (Cancer Diagnostics) Diabetes Care Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Retail Pharmacies Others |
| By Price Range | Low-End Devices Mid-Range Devices High-End Devices Others |
| By Component | Hardware Software Services Consumables Others |
| By Technology | Digital Technologies Traditional Technologies Hybrid Technologies AI-Enabled Technologies Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Healthcare Device Manufacturers | 60 | Product Managers, R&D Directors |
| Diagnostic Laboratories | 50 | Lab Managers, Quality Assurance Officers |
| Healthcare Providers (Hospitals & Clinics) | 80 | Chief Medical Officers, Procurement Specialists |
| Regulatory Bodies | 40 | Regulatory Affairs Managers, Compliance Officers |
| Patient Advocacy Groups | 40 | Patient Representatives, Healthcare Advocates |
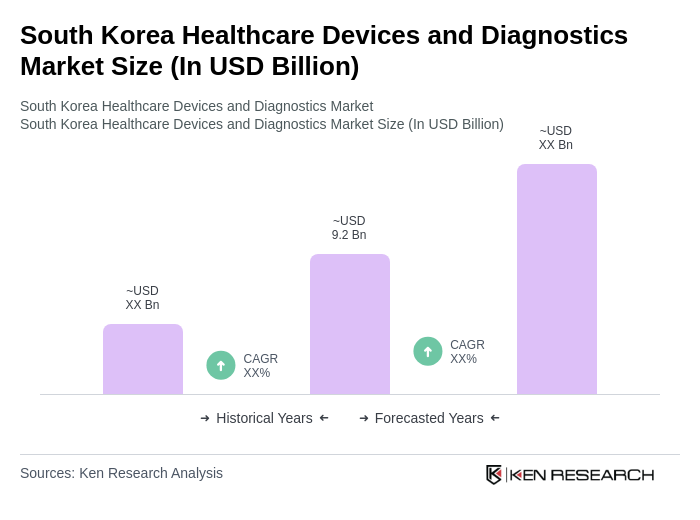
The South Korea Healthcare Devices and Diagnostics Market is valued at approximately USD 9.2 billion, driven by advancements in digital health, AI integration in diagnostics, an aging population, and increasing healthcare expenditure.